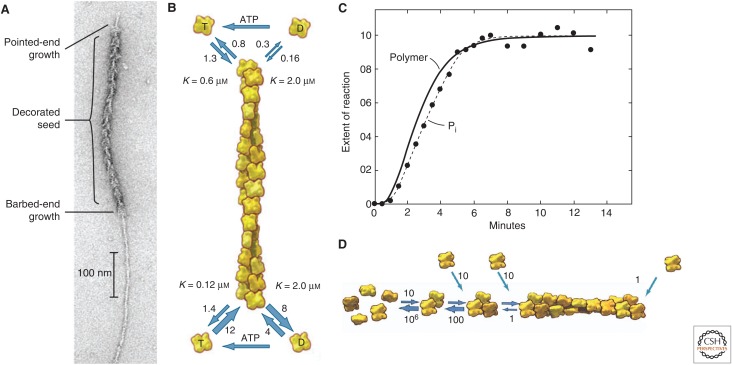
Figure 2.
Actin polymerization. (A) Electron micrograph of a negatively stained actin filament. A seed was first decorated with myosin heads and then allowed to grow bare extensions. Elongation was faster at the barbed end than at the pointed end. (B) Diagram showing the rate constants for actin association and dissociation at the two ends of an actin filament. The pointed end is at the top and the barbed end is at the bottom. Unit of association rate constants, µm−1 sec−1; unit of dissociation rate constants, sec−1. The K values are the ratios of dissociation rate constants to association rate constants, the critical concentrations for each of the four reactions. The horizontal arrows indicate the exchange of adenosine diphosphate (ADP) for ATP. (C) Time course of spontaneous polymerization of Mg-ATP–actin monomers. The solid line is the polymer concentration measured by the fluorescence of pyrene-labeled actin. The initial lag comes from slow spontaneous nucleation. The reaction reaches a steady state when the free actin monomer concentration reaches the overall critical concentration. Filled circles are the extent of hydrolysis of the bound ATP, which lags behind polymerization by a few seconds. (D) Mechanism of nucleation, showing monomers, a dimer, a trimer, and a filament, with estimates of the rate constants for each step. Unit of association rate constants, µm−1 sec−1; unit of dissociation rate constants, sec−1. (A,B,D, Adapted, with permission, from Pollard and Earnshaw 2007; C, reprinted from Pollard and Weeds 1984.)