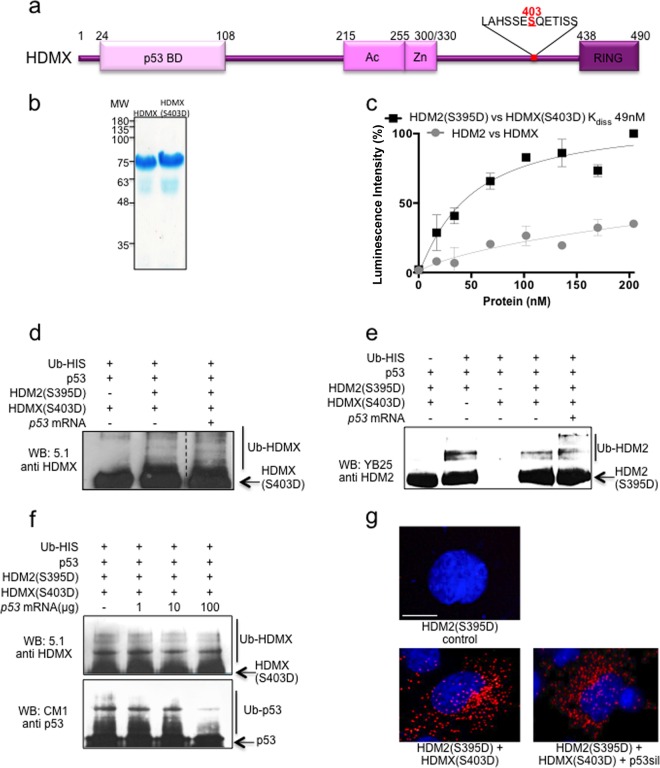
FIG 3.
HDM2(S395D)-p53 mRNA interaction does not inhibit ubiquitination of HDMX(S403D) and HDM2(S395D). (a) Cartoon illustrating major domains of HDMX. p53 BD, the p53 binding domain; Ac, acidic domain; Zn, zinc finger; RING, really interesting new gene domain. Serine 403 is indicated in red. (b) Recombinant purified HDMX and HDMX(S403D). (c) ELISA using a fixed amount of recombinant purified HDMX or phosphomimetic mutant HDMX(S403D) (10 ng/μl) and increasing amounts of HDM2 or HDM2(S395D) (0 to 20 ng/μl), respectively. The WT HDM2-HDMX proteins show very low affinity compared to that of the two proteins that mimic ATM-dependent phosphorylation. The figure represents averages and SD from five independent experiments. (d) In vitro HDM2(S395D) ubiquitination of recombinant HDMX(S403D) in the presence of p53 protein and with or without 100 ng p53 mRNA. The ubiquitination of HDMX(S403D) is not inhibited by the presence of p53 mRNA. (e) In vitro HDM2(S395D) autoubiquitination assay in the presence of p53 protein and HDMX(S403D) with or without p53 mRNA. In the presence of p53 mRNA, polyubiquitination increases, probably due to the inhibition of the interaction with p53 protein, which in the absence of mRNA is still able to interact with HDM2(S395D) and become ubiquitinated. (f) p53 mRNA dose-dependent in vitro ubiquitination of p53 and HDMX(S403D). (g) PLA using anti-HDMX and anti-HDM2. H1299 cells were transfected with the indicated constructs. Cell nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI) (blue).