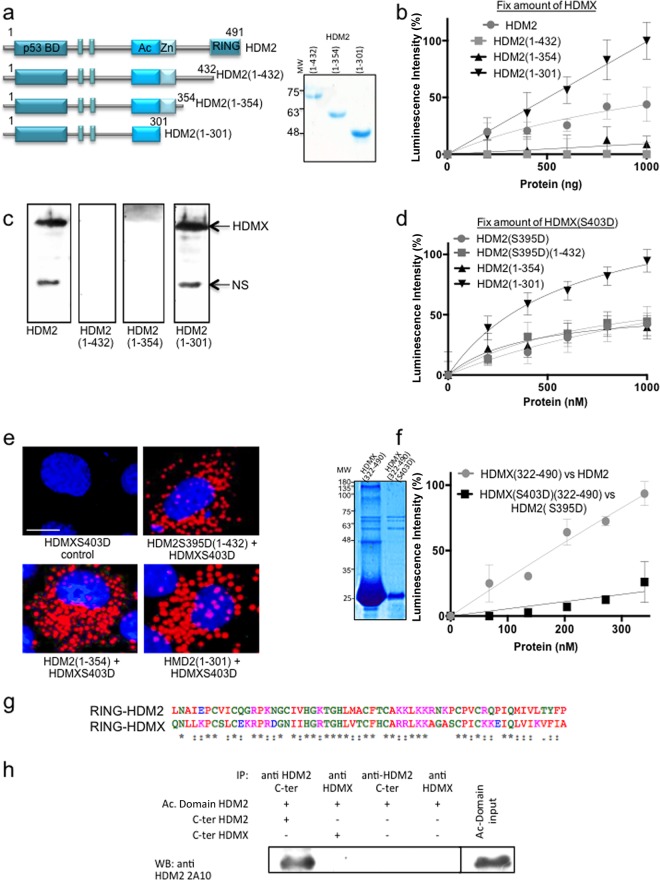
FIG 4.
HDMX-HDM2 interaction is independent of RING domains after DNA damage. (a) Diagrams of HDM2 stop constructs in the C terminus. Construct HDM2(1-432) (amino acids 1 to 432) lacks the RING C-terminal domain; construct HDM2(1-354) (amino acids 1 to 354) lacks the RING domain plus the IDR where S395 phosphorylation takes place. Construct HDM2(1-301) also is lacking the Zn finger domain (amino acids 1 to 301). Recombinant purified proteins are also shown. (b) ELISA using HDMX (10 ng/μl) and increasing amounts of HDM2, HDM2(1-432), HDM2(1-354), and HDM2(1-301) (from 0 to 10 ng/μl). Neither HDM2(1-432) nor HDM2(1-354) binds to HDMX. However, the smallest one, HDM2(1-301), binds even more strongly than full-length HDM2. (c) Far Western analysis of the interaction using the three constructs and full-length HDMX. NS, nonspecific. (d) Same as panel b but using HDMX(S403D), HDM2(S395D), and HDM2(S395D)(1-432) instead of the wild-type version of each protein. In this case, all of the constructs bind to HDMX(S403D) with affinity similar to that of the full-length HDM2(S395D), and HDM2(1-301) binds more strongly. (e) PLA using anti-HDMX and anti-HDM2. H1299 cells were transfected with the indicated constructs. Cell nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI) (blue). (f) ELISA using HDMX or HDMX(S403D) (10 ng/μl) and increasing concentrations of the C-terminal HDM2 or HDM2(S395D) (amino acids 322 to 491), respectively. The C terminus in the wild-type version binds the full-length HDMX more strongly than the phosphomimetic versions. The recombinant purified C-terminal domains are shown. (g) Alignment of the HDMX(432-491) and HDM2(431-490) RING domains shows more than 75% similitude and more than 45% identity (ESPript; http://espript.ibcp.fr). (h) Coimmunoprecipitation of C-terminal (C-ter) domain of HDMX(322-490) and Ac domain(241-335). The positive control is the Ac domain of HDM2 with the C terminus of HDM2(322-491).