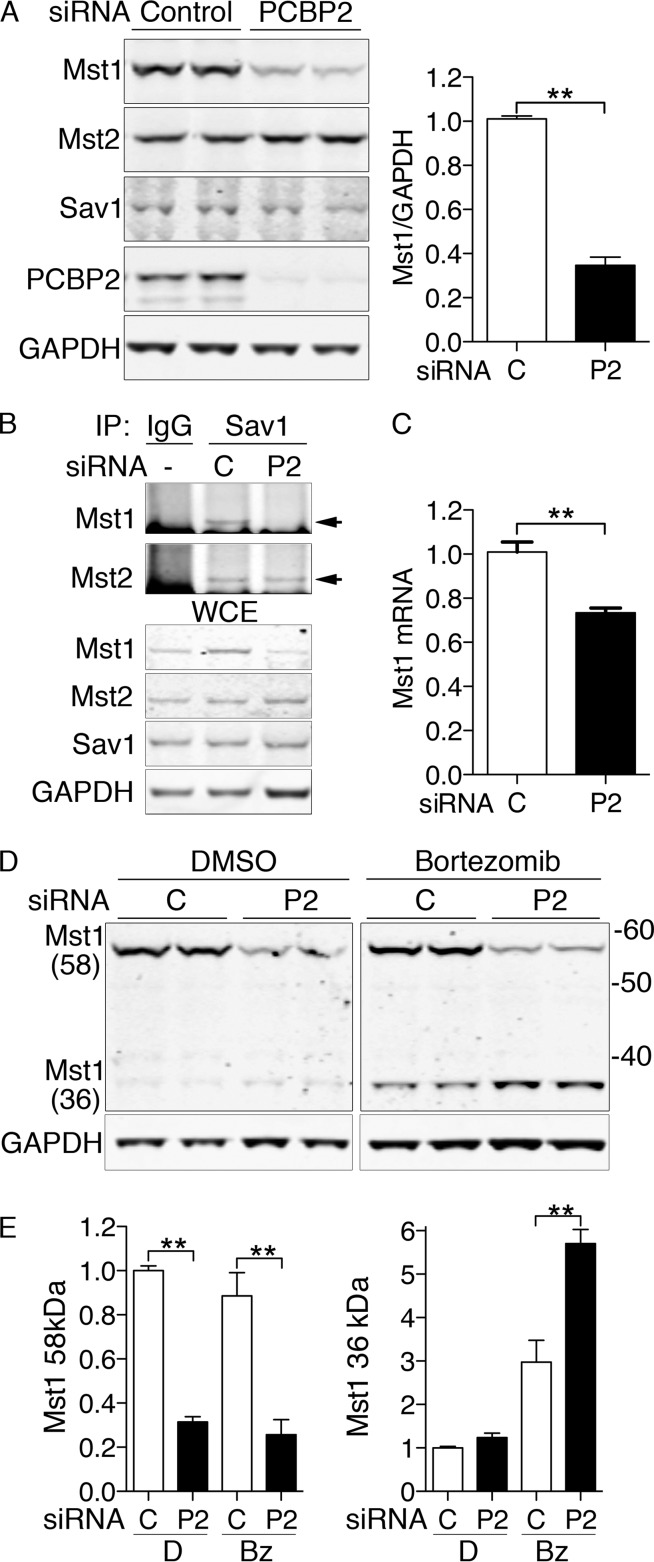
FIG 3.
Degradation of Mst1 with PCBP2 depletion in MCF10A cells. (A) Loss of Mst1 protein with PCBP2 depletion. MCF10A cells were transfected with nontargeting control or PCBP2-specific siRNAs and then analyzed by Western blotting. (Left) Paired biological replicates. (Right) Mst1 quantification. Ratios are normalized to control. (B) Loss of Mst1 from the Hippo complex with PCBP2 depletion. PCBP2 was depleted as for panel A. The lysates were subjected to IP with anti-Sav1 or rabbit IgG. (Top) Immune complexes were examined for Mst1 and Mst2. The arrows indicate specific Mst1/2 signal. (Bottom) WCE were analyzed by Western blotting for Mst1, Mst2, Sav1, and GAPDH. (C) Minor change in Mst1 mRNA transcripts with PCBP2 depletion. RNAs from control and PCBP2-depleted MCF10A cells were extracted, and Mst1 transcripts were measured by real-time PCR. Mst1 transcripts are presented relative to control-transfected cells. (D and E) Increased cleavage and degradation of MST1 with PCBP2 depletion. Cells were treated with either nontargeting or PCBP2-specific siRNAs for 48 h, followed by either diluent (dimethyl sulfoxide [DMSO]) or proteasome inhibitor (bortezomib; 25 nM) for 24 h. Mst1 was detected by Western blotting, and paired biological replicates are shown. (E) Mass is indicated in kDa. The long (58- Da) and short (36-kDa) forms of Mst1 were quantitated and analyzed separately. All experiments were replicated three times. The error bars indicate SEM. **, P < 0.0001.