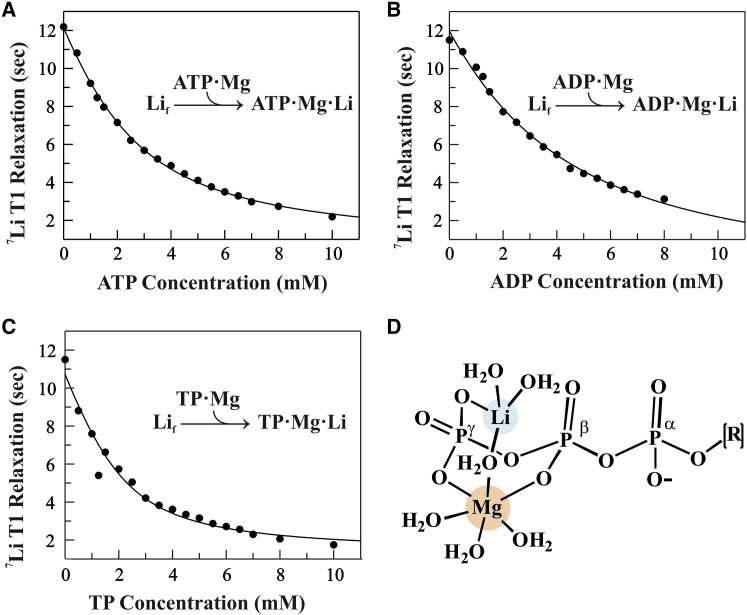
Figure 2.
Measured affinity of Li+ binding to ATP·Mg, ADP·Mg, and TP·Mg. 7Li T1 relaxation times (black circles) measured for 2 mM LiCl at 10°C as a function of the concentration of (A) ATP·Mg, (B) ADP·Mg, or (C) TP·Mg are shown. Fits using a quadratic binding equation (curves) yielded Li+ equilibrium dissociation constants, Kd: 1.60 ± 0.21 mM for ATP·Mg; 3.24 ± 0.56 mM for ADP·Mg; and 0.71 ± 0.23 mM for TP·Mg (reported uncertainties are standard errors calculated from least-squares fitting). Sample integrity was monitored using 31P NMR (Fig. S4). (D) A proposed molecular model for the ternary ATP·Mg·Li complex is shown. Mg2+ is 6-coordinate, with the β and γ phosphate oxygens replacing two water molecules. Li+ is 4-coordinate, with a γ phosphate oxygen replacing one water and a water bridge shared with Mg2+. To see this figure in color, go online.