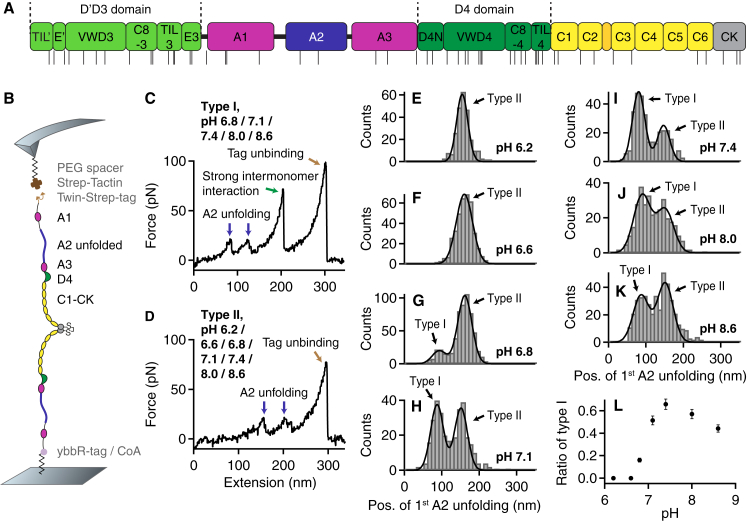
Figure 1.
Single-molecule force measurements on dimeric VWF A1-CK constructs under varied pH conditions. (A) Domain organization of mature VWF (residues 764–2813) as described in (1, 12). The positions of histidine residues are indicated by the black lines beneath. (B) Schematic of pulling dimeric A1-CK constructs (not drawn to scale). (C and D) Denoised force-extension traces of type I and type II characterized by A2 unfolding peaks (blue arrows) at low (type I) and high (type II) extension values, respectively. Type II traces were observed throughout the probed pH range, whereas type I traces, showing the force response of dimers that were initially firmly closed via the strong intermonomer interaction (13), were essentially obtained only at pH values of ≥6.8. For all pH values at which type I traces were observed, dissociation of the strong intermonomer interaction (green arrow) yielded unvaried characteristic length increments. The final peak (brown arrow) corresponds to tag unbinding, i.e., to dissociation of the specific Twin-Strep-tag/Strep-Tactin interaction and rupture of the probed molecule from the AFM tip (13, 34). (E and F) Unimodal distributions of the position of the first A2 unfolding peak obtained at pH 6.2 (E, n = 253) and 6.6 (F, n = 385). The distributions are well described by fits of Gaussian functions (solid lines). (G–K) Bimodal distributions of the position of the first A2 unfolding peak obtained at pH values of 6.8 (G, n = 586), 7.1 (H, n = 329), 7.4 (I, n = 317), 8.0 (J, n = 336), and 8.6 (K, n = 428). To estimate the ratio of type I and type II traces, the distributions were fitted with double Gaussian functions (solid lines). (L) Ratio of type I traces as a function of the pH. Error bars represent Poisson noise (1 SD). To see this figure in color, go online.