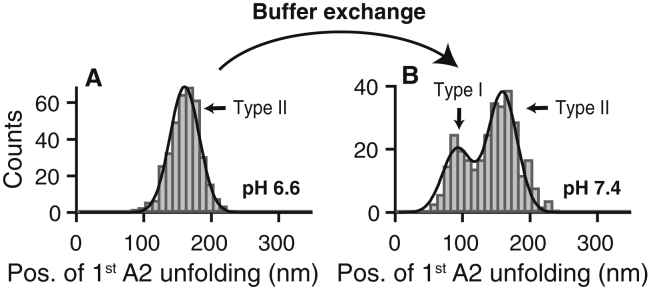
Figure 2.
Reversibility of the pH-dependent mechanisms affecting the formation of the strong intermonomer interaction in VWF’s dimeric subunits. (A) Unimodal distribution of the position of the first A2 unfolding peak in force-extension traces of A1-CK dimers measured at pH 6.6 (same histogram as shown in Fig. 1F, n = 385), indicating that only type II traces were observed. (B) Histogram of the position of the first A2 unfolding peak, obtained after buffer exchanging immobilized proteins to a buffer solution with pH 7.4 (n = 323) and with the same cantilever that was used at pH 6.6. The bimodality of the distribution indicates that a significant number of type I traces were observed, proving that the molecular mechanisms that critically affect the pH-dependent formation of the strong intermonomer interaction are largely reversible. Fitting a double Gaussian (solid line) yielded a ratio of (35 ± 3)% type I traces. To see this figure in color, go online.