Figure 3.
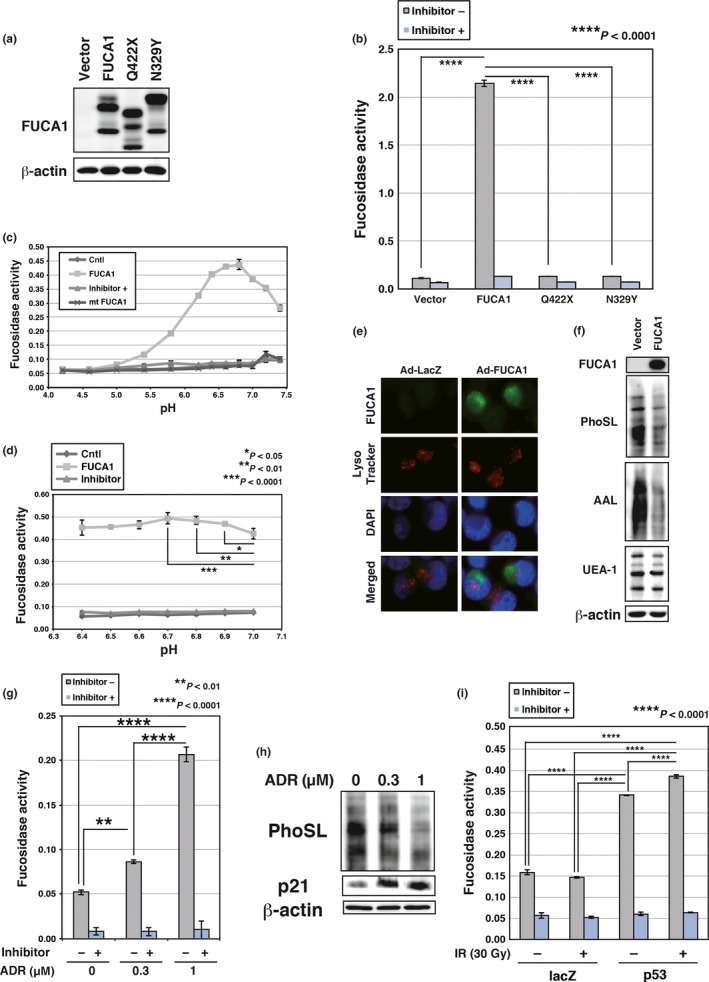
Fucosidase, α‐l‐1 (FUCA1) is active at physiological pH. (a) COS7 cells were transduced with FUCA1, N329Y, Q422X, or control vector. Western blotting was carried out using whole cell lysates. FUCA1 protein has several glycosylation sites and FUCA1 undergoes glycosylation (predicted N‐glycosylation sites N241, N251, N268, and N382; N268 is an identified N‐glycosylation site). The difference in the protein size between wild‐type and N329Y proteins may be the result of abnormal glycosylation of the N329Y mutant protein. (b) Enzyme assay was carried out with (+) or without (−) an inhibitor of FUCA1, using lysates of COS7 cells transduced with FUCA1, N329Y, Q422X, or control vector. The reaction was carried out for 3 h at 37°C in PBS. (c,d) FUCA1‐FLAG and N329Y‐FLAG were purified by immunoprecipitation from whole cell lysates of 293T cells transduced with empty vector (Cntl), FUCA1‐FLAG, or N329Y‐FLAG. Enzyme assays were carried out with or without an inhibitor of FUCA1, using purified wild‐type or mutant (mt) FUCA1. The reaction was carried out for 9 h (c) or 11 h (d) at 37°C in 0.1 M citrate/0.2 M sodium phosphate (McIlvaine) buffer at the indicated pH. Significant enzymatic activities (P < 0.05) were detected between pH 5.4 and 7.4 (c). (e) Subcellular localization of FUCA1 was analyzed. H1299 cells were infected with control (Ad‐LacZ) or FUCA1 (Ad‐FUCA1) expressing adenoviruses. Cells were harvested 37 h post‐infection. Lysosomes were stained with LysoTracker Red and nuclei were stained by DAPI. (f) H1299 cells were infected with control or FUCA1‐expressing adenoviruses. Cells were harvested 62 h post‐infection. Lectin blotting and Western blotting was undertaken using whole cell lysates of H1299. AAL, Argiope aurantia lectin; PhoSL, Pholiota squarrosa lectin; UEA‐1, Ulex europaeus lectin. (g) Enzyme assays were undertaken with or without an inhibitor of FUCA1, using lysates of MRC5 cells subjected to adriamycin (ADR; 0.3 or 1 μM). Cells were harvested 120 h post‐ADR treatment. The enzyme assay reaction was carried out for 52 h at 37°C in PBS. (h) MRC5 cells were subjected to ADR (0.3 or 1 μM). Cells were harvested 120 h post‐ADR treatment. Lectin blotting and Western blotting were carried out using whole cell lysates of MRC5. (i) Enzyme assays were carried out with or without an inhibitor of FUCA1, using lysates of H1648 cells infected with control or p53‐expressing adenoviruses. Cells were subjected to γ‐ray irradiation (IR; 30 Gy) 24 h after virus infection. Cells were harvested 60 h post‐irradiation. The enzyme assay reaction was carried out for 24 h at 37°C in PBS.
