Figure 1.
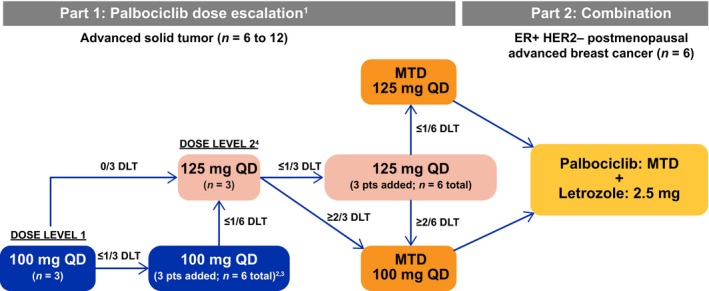
Design of this phase I study of palbociclib in Japanese patients. 1In principle, two dosages (100 mg once daily [QD] and 125 mg QD) were examined; where necessary, additional/lower dose levels (75 mg QD, dose level −1) were explored. 2If two or more patients of three to six patients at dose level 1 experienced a dose‐limiting toxicity (DLT) during cycle 1, the dose was considered intolerable and a lower dose (75 mg QD, dose level −1) was used. 3If no further DLTs occurred in the three additional patients such that only one of six patients at dose level 1 experienced DLT(s) during the first cycle, then the dose was escalated to dose level 2 (125 mg QD) in a subsequent cohort of patients. 4If two or more patients of three to six patients at dose level 2 experienced a DLT during the first cycle, the dose was de‐escalated to dose level 1 (100 mg QD) unless six patients were enrolled and evaluated at dose level 1 at that time. ER+, estrogen receptor‐positive; HER2−, human epidermal growth factor receptor‐negative; MTD, maximum tolerated dose; pts, patients.
