Fig. 3.
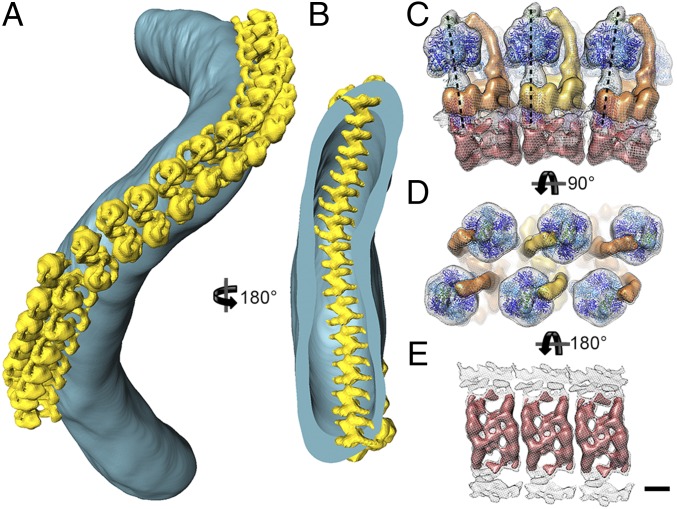
Macromolecular organization of ATP synthase dimers in P. tetraurelia cristae. (A) Surface representation of an isolated crista tube (blue) showing the in situ organization of ATP synthase dimers (yellow). The ATP synthase dimers form helical arrays around the outer perimeter of the coiled tubular crista. (B) Rotated clipped view of A showing the ribbed arrangement of ATP synthase dimers on the luminal membrane surface. The location of the ATP synthase dimers was determined by positioning the subtomogram average into the tomographic volume using the positions and orientations determined during alignment. (C) Side view, (D) matrix view, and (E) luminal view of three ATP synthase dimers in the helical array. The F1 subcomplexes form a zigzag pattern (D), whereas the intracrista regions give the luminal view a ribbed appearance (E). Neighboring dimers are rotated by 8° in the direction of the row (black dashed lines, C). C–E were generated by fitting the central dimer density into the neighboring dimer densities present in the subtomogram average (see Fig. S6). (Scale bar, 5 nm.) See Movie S3.