Figure 4.
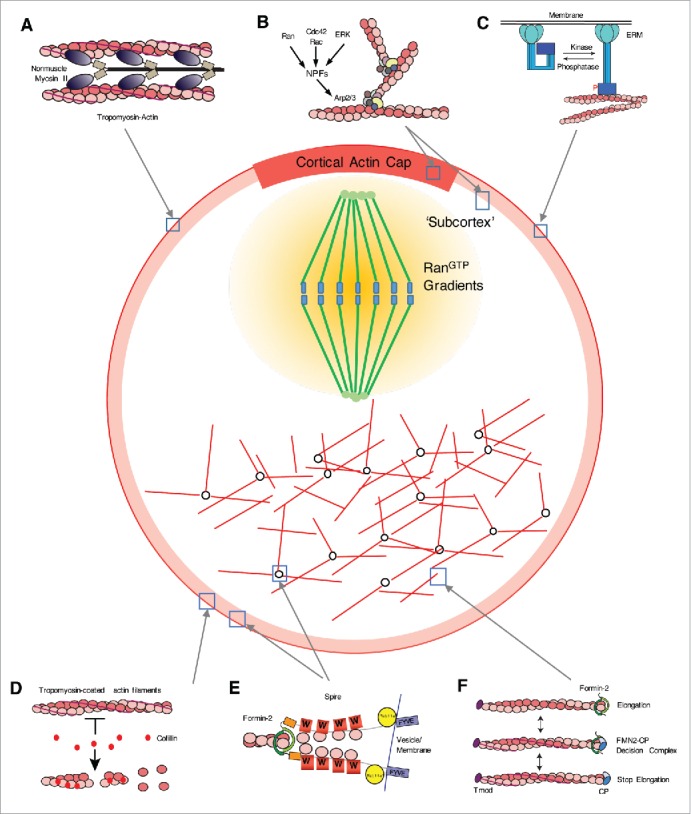
Various molecular events governed by ABPs in maturing oocytes. Localizations of ABPs in the oocyte are also indicated. Actin in oocytes were marked as red. (A) Actomyosin contraction between actin filaments and non-muscle myosin II is responsible for maintaining the cortical tension of the oocyte cortex.12,13,123 During oocyte maturation, delocalization of myosin II decreases cortical tension and is crucial for spindle migration.12 (B) ARP2/3-mediated actin polymerization is responsible for the formation of the cortical actin cap67,73,76 and cortex thickening during oocyte maturation.12,13 Small GTPases including RAC1,72 CDC42,67 and RANGTP/GDP76 are implicated in the activation of NPFs, which in turn activate the ARP2/3 complex. The MOS-MAPK pathway173 is also involved in triggering WAVE2-dependent ARP2/3-mediated actin polymerization.12 (C) ERM proteins localize in the oocyte cortex, act as link between the plasma membrane and cortical actin, and are involved in maintaining cortical tension.123 ERM protein is activated by phosphorylation on the C-terminal tail domain and deactivated by dephosphorylation.122 A recent study suggests that p-ERM is excluded at the cortex near the approaching spindle, which is mediated by RAN-based signaling.77 (D) In the oocyte cortex, Tpm3.1 is present with cortical actin and protects against depolymerization by cofilin.84 Cofilin activity in oocytes is regulated by phosphorylation by ROCK-LIM kinase cascades.83 (E) Formin-2 and spire are both responsible for the generation of cytoplasmic actin mesh or cortical actin10,11,38,39 in mammalian oocytes. Biochemical results55,59,154 suggest that both proteins synergistically promote actin nucleation. WH2 domain (marked as W) recruit monomeric actin. The presence of a potential Rab-binding domain and membrane-binding FYVE domain in the C-terminal part of spire56 suggests the possibility that spire mediates the recruitment of formin-2 in vesicles and the membrane in cooperation with Rab11a.38 (F) Formin-2 and heterodimeric CP compete against each other at the fast-growing filament end and regulate actin elogation100,101 Because the length of actin filaments generated by formins is much longer than that of cytoplasmic actin mesh10,37,101, some mechanism must regulate the elongation of actin filaments by formin-2. Impairment of CP in mouse oocytes decreases cytoplasmic actin density,99 suggesting that CP regulates actin mesh.
