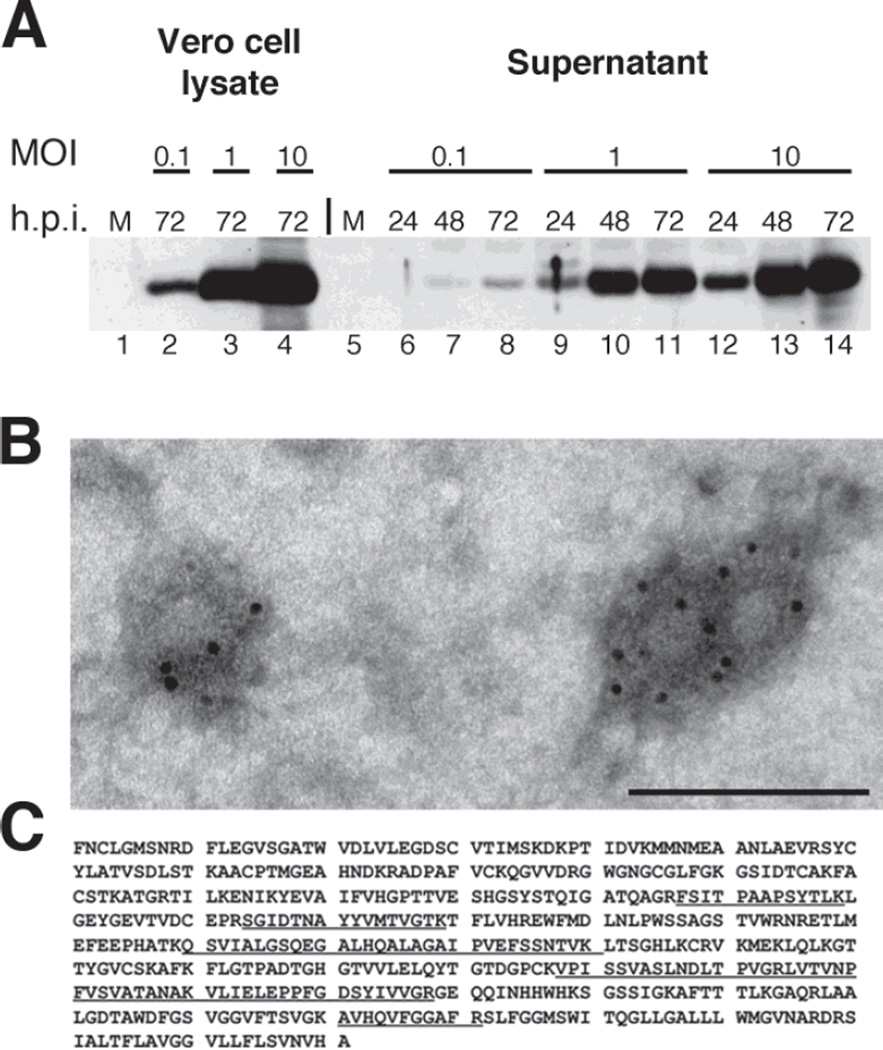
Figure 3. Assembly and properties of virus-like particles in d106-WNV virus-infected cells.
A. Kinetics of WNV protein expression and VLP release. A. Vero cells were mock-infected or infected with d106-WNV virus at a range of MOIs (0.1, 1.0 or 10 PFU/cell) and harvested at 24, 48, or 72 hpi. Lysate (10% of sample total) and clarified supernatant (2% of sample total) were separated by SDS-PAGE, transferred to membrane, and probed with anti-E monoclonal antibody. The position of the 48.8 kDa ladder band is indicated. B. Electron micrograph of VLPs. WNV VLPs were diluted in Tris-buffered saline and adsorbed for 1 minute to a carbon coated grid that had been made hydrophilic by a 30 second exposure to a glow discharge. Samples were blocked with 1% BSA and incubated with a mouse anti West Nile Virus Envelope protein (Chemicon) for 30 minutes. After washing, samples were incubated with a rabbit anti mouse bridging antibody and protein A-gold (5nm) for 20 minutes. Samples were washed with PBS and water. Excess liquid was removed with a filter paper, and the samples were stained with 0.75% uranyl formate for 30 seconds. After removing the excess uranyl formate with a filter paper, the grids were examined in a JEOL 1200EX Transmission electron microscope, and images were recorded with an AMT 2k CCD camera. Scale bar: 100 nm. C. Identification of Peptides from E Protein in Extracellular Particles. Partially purified VLPs were resolved by SDS-PAGE, and the Coomassie blue-stained band corresponding to E was analyzed by mass spectrometry. The E-specific peptides identified by mass spectrometry are underlined.