Figure 3.
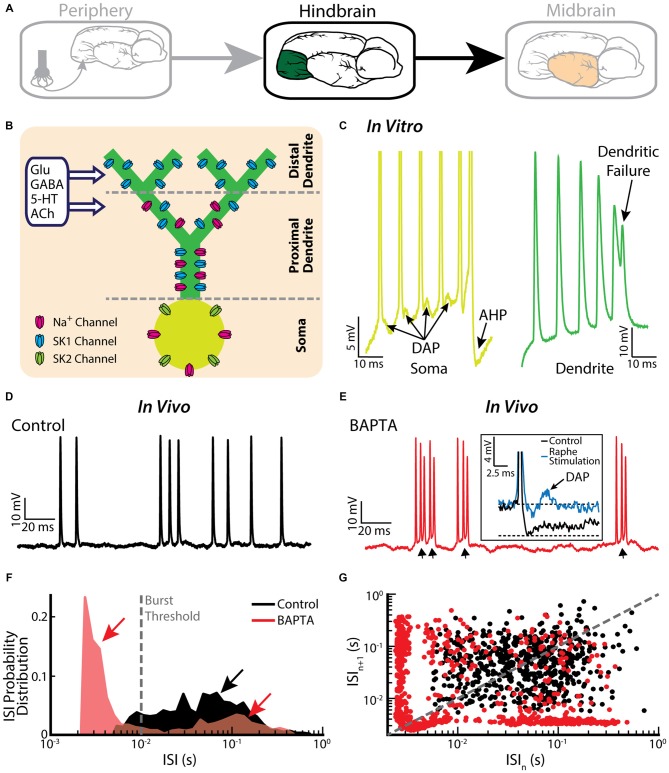
Bursting in neurons in the hindbrain ELL. (A) ELL pyramidal cells receive input from the electrosensory primary afferents and project to the midbrain. (B) Schematic showing the distribution of sodium (Na+, magenta), and two subtypes of small-conductance potassium (blue: SK1; green: SK2) channels. Na+ channels are located in the soma as well as the proximal dendrite, SK1 channels are located in the proximal and distal dendrite, whereas SK2 channels are only expressed in the soma. Neuromodulators, such as serotonin (5-HT) and acetylcholine (ACh), influence spiking. (C) A somatic and dendritic burst of spikes recorded separately in two cells (somatic spikes are truncated). The slowdown in dendritic spike repolarization is due to inactivation of a dendritic K+ conductance and results in a potentiation of the somatic depolarizing afterpotential (DAP; arrows). When the DAP reaches threshold for a high-frequency spike doublet, the second spike fails to backpropagate. This allows the afterhyperpolarization (AHP) to terminate the burst. (D) Example in vivo recording of an ELL pyramidal cell under control conditions. (E) The same neuron as in (E) displays bursting after treatment with the Ca2+ chelator BAPTA. The arrows indicate the ramp depolarizations. Inset: a DAP is seen after electrically stimulating serotonergic pathways (blue line). (F) ISI distribution under control condition (black) and after BAPTA treatment (red) showing a decrease in the cell’s absolute refractory period and the emergence of a second peak (red arrows). The burst threshold used to segregate bursts and isolated spikes for ELL pyramidal cells was 10 ms. (G) ISI return map under control condition (black) and after BAPTA treatment (red) showing a transition to a bursting regime. The data plotted in (D–G) are from Toporikova and Chacron (2009).