This is the first work to show that CaMKII activation and subsequent AMPA receptor insertion mediates stimulus-specific odor memory and its blockade prevents such memory, therefore supporting the hypothesis that CaMKII serves as the synaptic tag mediating input specificity in natural memory formation as it has been shown to do in long-term potentiation.
Keywords: CaMKII, cAMP, PKA, CREB, AMPA receptor, olfactory bulb, early odor preference learning
Abstract
After naturalistic odor preference training, Ca2+/calmodulin-dependent protein kinase II (CaMKII) was rapidly phosphorylated in the olfactory bulb, specifically in the odor encoding regions of the glomerular layer and external plexiform layer. Intrabulbar CaMKII antagonist experiments revealed that CaMKII supports short- and long-term preference memory formation. With bulbar PKA activation as the unconditioned stimulus odor preferences could be induced despite CaMKII blockade, but now odor specificity was lost, with odor preference generalizing to an untrained odor. Odor-specific learning was associated with increased membrane-associated AMPA receptors, while nonspecific odor preference was not. Thus CaMKII activation provides a tag to confer stimulus specificity as well as supporting natural odor preference learning.
NEW & NOTEWORTHY
This is the first work to show that CaMKII activation and subsequent AMPA receptor insertion mediates stimulus-specific odor memory and its blockade prevents such memory, therefore supporting the hypothesis that CaMKII serves as the synaptic tag mediating input specificity in natural memory formation as it has been shown to do in long-term potentiation.
long-term potentiation (LTP) is widely accepted as a cellular model for learning. A basic property of LTP is input specificity (Bliss and Collingridge 1993). The synaptic tagging and capture hypothesis is a framework that explains input specificity. Newly synthesized plasticity-related products are captured by tagged synapses and support the creation of input-specific synaptic changes (Frey and Morris 1997). Ca2+/calmodulin-dependent protein kinase II (CaMKII) is proposed to mediate synaptic tagging in mammalian brains because of its prolonged, synapse-specific activation (Sanhueza and Lisman 2013).
CaMKII has been proposed as the integral component in mediating LTP and memory. Injection of inhibitors of CaMKII or genetic disruption of CaMKII blocks the ability to generate LTP (Malenka et al. 1989; Silva et al. 1992b) and impairs hippocampus-dependent spatial learning (Silva et al. 1992a; Vaynman et al. 2007). Activation of CaMKII leads to enhancement of synaptic transmission, and LTP is occluded by increasing the concentrations of constitutively active CaMKII (Pettit et al. 1994). The role of CaMKII as a synaptic tag that mediates synapse-specific LTP has been shown in hippocampal CA1 of αCaMKII-T286A mutant mice (Villers et al. 2014). Several forms of LTP can be induced in these mice but at the expense of synaptic input specificity. In the absence of αCaMKII autophosphorylation, LTP is not restricted to stimulated synapses (Villers et al. 2014). However, the role of CaMKII in the stimulus specificity of natural learning and memory has not been addressed explicitly.
Early odor preference learning occurs in week-old rat pups receiving convergent odor input and tactile stimulation. In these pups the hippocampus is immature and does not participate in odor learning (Raineki et al. 2010). Norepinephrine release from locus coeruleus to the olfactory bulb (OB) and the piriform cortex during tactile stimulation, which uniquely recruits high levels of release at this early age, mediates the unconditioned stimulus (Sullivan et al. 2000; Yuan et al. 2014). Consequent activation of β-adrenoceptors leading to increases in cytoplasmic cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) and recruitment of the nuclear cAMP-response element binding protein (CREB) (the cAMP/PKA/CREB cascade) in the OB is both necessary and sufficient for one-trial odor preference learning (McLean et al. 1999; Yuan et al. 2003), which creates a protein synthesis-dependent long-term memory (LTM) lasting 24 h (Grimes et al. 2011). Odor preference short-term memory (STM) occurs with the same training but does not require the PKA/CREB components of the cascade (Grimes et al. 2012); instead it depends on exchange protein directly activated by cAMP (Epac) signaling (Grimes et al. 2015), an alternate cAMP effector pathway. Epac activation can act as an unconditioned stimulus to induce both 3-h and 24-h odor preference learning (Grimes et al. 2015). Thus normal odor preference learning in rat pups is supported by cAMP activation of both PKA and Epac (Grimes et al. 2015).
On the other hand, stimulus specificity of the odor learning is likely mediated by calcium signaling activated upon coordinated inputs of an odor and norepinephrine. CaMKII is recruited by calcium influx and critically involved in postsynaptic membrane trafficking of AMPA receptors (AMPARs) (Hayashi et al. 2000). We have proposed that the main output mitral cells (MCs) are the substrate for learning (Yuan et al. 2003) and the olfactory nerve (ON)-to-MC synapse is the first synapse encoding odor learning (Lethbridge et al. 2012; Yuan et al. 2014). Different odors activate distinct sets of glomeruli where ON input terminals form synapses with MC primary dendrites. Thus each odor activates distinct sets of glomeruli and ON-MC synapses. The present experiments assess the role of CaMKII in STM and LTM and in the odor stimulus specificity of memory.
MATERIALS AND METHODS
All experimental procedures were approved by the Animal Care Committee at Memorial University and adhered to Canadian Council on Animal Care guidelines. Sprague-Dawley rats were bred for male and female pups. Dams were maintained under a 12-h reverse light-dark cycle at 22°C with ad libitum food and water. Day of birth was considered postnatal day 0 (PND0).
Early odor preference training and testing.
Bilateral OB cannulas were implanted on PND5 following standard methodology (Grimes et al. 2012). Pups were anesthetized via hypothermia, and custom-made guide cannulas were inserted into the OB and fixed to the bone overlying the OBs. On PND6 animals received bilateral infusions of 1 μl of a CaMKII antagonist, KN-62 (3.6 μg/μl) (Vaynman et al. 2007), dissolved in 75% dimethyl sulfoxide (DMSO) and 25% saline, or vehicle alone into each OB 10 min prior to odor-preference training. A separate cohort was infused with KN-62, vehicle, tatCN21 (a highly specific CaMKII inhibitory peptide, 2.0 µg/µl in saline), or its scrambled control (Liu et al. 2014) or vehicle immediately after odor training. In other cannulated pups, Sp-cAMPs, a cAMP/PKA activator (18 μg/μl, dissolved in 1 μl saline) (Grimes et al. 2012), or the combination of Sp-cAMPs and KN-62 (in the same vehicle as KN-62 alone) was infused into the OB before training.
Naturalistic odor preference training was given on PND6 by placing pups over peppermint-scented bedding for 10 min, during which they were stroked with a paintbrush to mimic maternal care for alternating 30-s intervals (O/S). Control pups on scented bedding did not receive stroking (O/O).
Pups were tested for odor preference either 3 h (STM) or 24 h (LTM) later in a stainless steel test box placed on top of two bedding boxes (one peppermint-scented, one unscented bedding) separated by a 2-cm neutral zone. Pups were placed on the neutral zone and allowed to move freely for 1 min. After 1 min, they were removed from the testing box to rest for 1 min. They were given five testing trials. The percentage of time spent over peppermint bedding over total time for both beddings was calculated. A subset of pups were tested for vanillin preference 1 h after peppermint test with the same odor dilution and protocol. Cannula locations were verified by infusions of 4% methylene blue followed by OB dissection. All included pups had functioning cannulas and correct OB placements. Dye inspection and Nissl staining revealed that dye diffusion was confined to the OB (n = 6; see Fig. 2E).
Fig. 2.
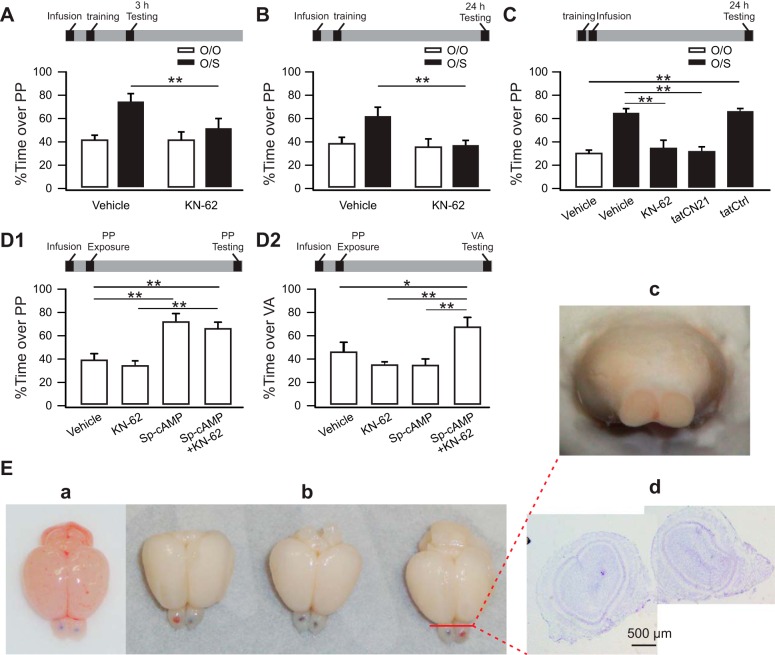
The roles of CaMKII in short-term memory, long-term memory, and input specificity of long-term memory. A: % of time spent over the peppermint (PP) bedding in KN-62-infused and control rat pups 3 h after odor preference training. Infusions were done 10 min before training. B: % of time spent over PP in KN-62-infused and control rat pups 24 h after odor preference training. Infusions were done 10 min before training. C: % of time spent over PP in pups infused immediately after training. D1 and D2: % of time spent over the PP or vanillin (VA) bedding in rat pups infused with KN-62, Sp-cAMPs, Sp-cAMPs+KN-62, or vehicle. O/O, odor only; O/S, odor+stroking. *P < 0.05; **P < 0.01. E: examples of dye infusion in the OBs. a: A rat brain directly taken after infusion. b: Three brains taken following perfusion after the dye infusion. c: A cryostat cutting plane showing that no dye is observed at this level. d: Corresponding Nissl staining at plane c.
Synaptic membrane extraction and Western blot.
On PND6, rat pups were trained and decapitated and OBs were collected and flash frozen on dry ice at ∼5–10 min, 30 min, or 2 h after training. An untreated naive group was included.
Purification of synaptic membrane followed an established protocol (Mukherjee et al. 2015). Tissue samples were homogenized in ice-cold sucrose buffer (300 μl) containing (in mM) 320 sucrose, 10 Tris (pH 7.4), 1 EDTA, and 1 EGTA, with 1× complete protease inhibitor mixture and phosphatase inhibitor mixture (Roche). The homogenized samples were centrifuged at 1,000 rpm for 10 min. The supernatant was spun at 10,000 rpm for 30 min to obtain a pellet, which was resuspended in 120 μl of sucrose buffer with a mixing/grinding pestle in the microfuge tube. Eight volumes of a nonionic detergent Triton X-100 buffer [final 0.5% (vol/vol)] were added for extraction. The Triton X-100 buffer contained (in mM) 10 Tris (pH 7.4), 1 EDTA, and 1 EGTA, with 1× protease and phosphatase inhibitors. The suspension was incubated at 4°C for 35 min with gentle rotation and then centrifuged at 32,000 rpm for 30 min.
The pellet (postsynaptic densities and synaptic junctions insoluble in Triton X-100) (Cotman and Taylor 1972) was resuspended in 100 μl of TE buffer containing 100 mM Tris (pH 7.4), 10 mM EDTA, 1% SDS, and 1× protease and phosphatase inhibitors, sonicated, boiled for 3 min, and stored at −80°C until final assays. Protein concentrations were determined with a BCA protein assay kit (Pierce). The lysate volume required to make 35 μg of protein for each sample was calculated. A total of 100 μl of lysate solution, sample buffer (0.3M Tris·HCl, 10% SDS, 50% glycerol, 0.25% bromophenol blue, 0.5M dithiothreitol), and dH2O was prepared and boiled for 2 min at 100°C. Samples were loaded into lanes of a 7.5% SDS-PAGE gel along with a protein ladder (Thermo Scientific). Sample separation occurred through SDS-PAGE, followed by transference to a nitrocellulose membrane.
A phosphorylated CaMKII (pCaMKII) antibody (1:2,000; Abcam) and a control β-actin antibody (1:5,000, blocked in 5% skim milk; Cell Signaling) were used to measure pCaMKII levels. Membranes were incubated in primary antibody overnight at 4°C in a continuous shaker. Membranes were washed three times for 5 min each with 1× TBST. HRP-conjugated secondary antibodies were applied after the wash (1:10,000, anti-rabbit; Pierce) for 1 h, and membranes were washed again with 1× TBST three times for 10 min each. Then membranes were washed in enhanced chemiluminescence Western blotting substrate (Pierce), developed on X-ray film, and scanned with an image scanner (CanoScan LiDE 200). Membranes were then stripped of pCaMKII for 20 min with Thermo Scientific Restore Western Blot Stripping Buffer at 50°C, blocked for 1 h with 5% milk in 1× TBST, and incubated overnight with β-actin antibody at 4°C, followed by the secondary antibody as described above. The optical density (OD) of each band was measured with ImageJ software. Each sample was normalized to the corresponding β-actin band.
For assessing the influence of CaMKII on AMPAR insertion, OB samples were collected 24 h after odor training. GluA1 antibody (1:7,000) was used with the extract of the synaptic fraction. Membranes were cut horizontally at the 72 kDa level; the upper portion was probed with a rabbit antibody for GluA1 (1:7,000, blocked in 5% milk; Cell Signaling Technology) subunits, and the lower portion was probed for β-actin (1:5,000).
Immunohistochemistry.
CaMKII and pCaMKII cellular expression patterns in OB were investigated by perfusing pups 5–10 min after O/S training. To highlight regional activation by the conditioned odor, intra-animal controls were used. Rat pups underwent single-naris occlusion 10 min before the training with a nose plug. The nose plug was made of a 2-mm polyethylene 20 tubing with a knotted thread inside (Fontaine et al. 2014). After training animals were deeply anesthetized and perfused transcardially with ice-cold saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed, postfixed for 1 h, and then immersed in 20% sucrose solution overnight at −4°C. Sucrose-soaked brains were cut coronally in a cryostat at −20°C (30 μm) and slide-mounted. Primary antibodies to CaMKII and pCaMKII (Abcam) were made fresh at 1:1,000 in PBS with 0.2% Triton X-100, 0.02% sodium azide, and 2% normal goat serum and then applied to slides and left overnight at 4°C in a humidified chamber. The next day, slides were incubated in a biotinylated secondary antibody (goat anti-rabbit, Vectastain Elite) followed by a diaminobenzidine tetrahydrochloride reaction. Slides were dehydrated and coverslipped with Permount.
Quantitative comparisons of pCaMKII levels were made between occluded and spared OBs. Images were taken by a Leica DFC495 camera, and analysis was conducted with ImageJ software. Five to eight OB sections from the rostral to caudal range of the OB were analyzed, and results were averaged for each animal. An OD reading of the background was taken from the center of the OB devoid of pCaMKII staining. A region of interest (ROI) was manually traced in both the dorsolateral and ventromedial regions of the glomerular layer and the external plexiform layer. The relative OD (ROD) was calculated as ROD = (OD of background − OD of ROI)/OD of background. The darker staining yields a smaller OD value. A paired Student's t-test was used to compare the occluded and spared OB staining within the same animals.
RESULTS
CaMKII is activated specifically in the odor coding region in the olfactory bulb after early odor preference training.
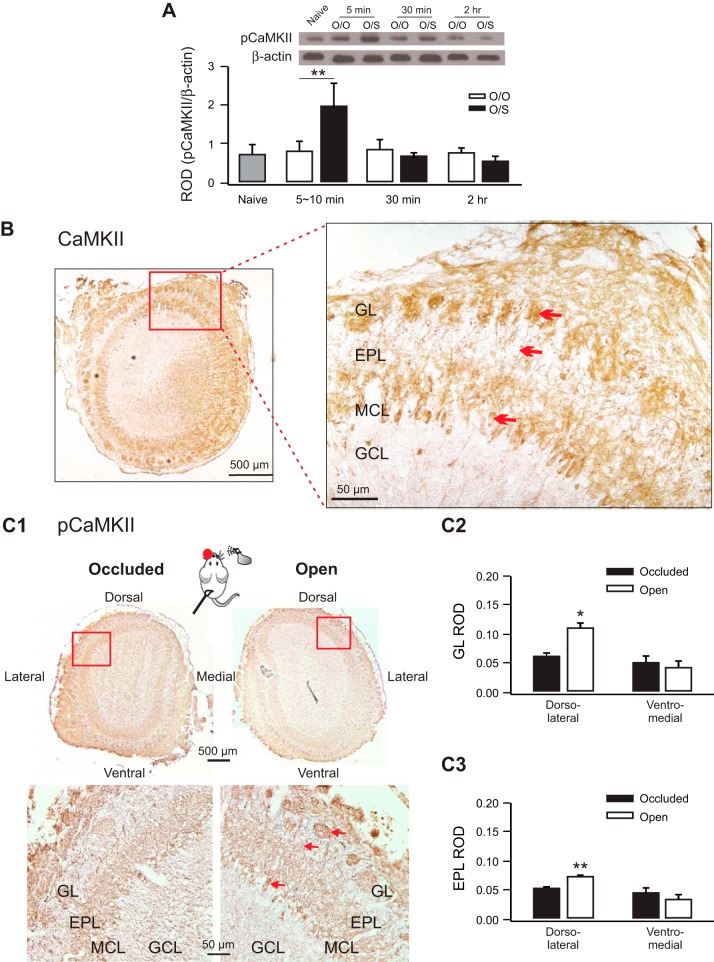
In the first set of experiments, we examined CaMKII activation following early odor preference training with Western blotting. A group (O/O vs. O/S) × time (5 min, 30 min, 2 h) two-way ANOVA revealed a significant interaction between time and group (F2,30 = 3.87, P = 0.03; Fig. 1A). Post hoc Fisher tests demonstrated elevated pCaMKII in O/S pups killed ∼5–10 min after training compared with O/O pups at the same time (t = 3.43, P < 0.01), confirming CaMKII activation in the postsynaptic density (PSD) as a response to associative odor preference training.
Fig. 1.
Early odor preference learning in rats induces transient phosphorylation of CaMKII in MC synapses of the olfactory bulb. A: relative optical density (ROD) of phosphorylated CaMKII (pCaMKII) over β-actin. O/O, odor only; O/S, odor+stroking. B: immunohistochemistry of CaMKII expression in the olfactory bulb. Arrows indicate a stained MC body and its apical dendrite going into a glomerulus. C: pCaMKII expression in the olfactory bulbs from single-naris-occluded pups. C1: example images of the pCaMKII staining in occluded vs. spared OB. Arrows indicate a stained MC body and its apical dendrite going into a glomerulus. C2: ROD of pCaMKII in the glomerular layer. C3: ROD of pCaMKII in the external plexiform layer. GL, glomerular layer; EPL, external plexiform layer; MCL, MC layer; GCL, granule cell layer. *P < 0.05; **P < 0.01.
The immunohistochemistry staining pattern showed that both CaMKII and pCaMKII are present in trained pup OB in the ON layer, the glomerular layer, the MC layer, and the external plexiform layer (n = 4 from non-naris-occluded pups and n = 3 from naris-occluded pups; Fig. 1, B and C). MC somas and apical dendrites including terminals in the glomerulus were clearly stained. Little staining was observed in the granule cell layer. CaMKII staining appeared to be uniform in all regions of the OB (Fig. 1B). Single-naris occlusion permitted detection of the pCaMKII activation pattern specific to the peppermint odor conditioning. In the dorsolateral region of the OB, where CREB phosphorylation in the MCs has been shown to increase after peppermint conditioned learning (McLean et al. 1999), pCaMKII was significantly higher in both the glomerular layer (spared naris 0.112 ± 0.007 vs. occluded naris 0.064 ± 0.003, t = 9.07, P < 0.05) and the external plexiform layer (spared naris 0.752 ± 0.001 vs. occluded naris 0.546 ± 0.001, n = 3; t = 17.8, P < 0.01; Fig. 1C). However, in the ventromedial region, neither glomerular layer nor external plexiform layer pCaMKII staining was significantly different between the occluded and spared OB (P > 0.05, Fig. 1C).
Causal roles of CaMKII in short-term and long-term odor preference memory.
The second set of experiments asked whether CaMKII is causal in odor preference learning by blocking bulbar CaMKII during training and assessing odor preference acquisition. One-way ANOVAs revealed group effects for both 3-h STM (F3,27 = 6.72, P = 0.002; Fig. 2A) and 24-h LTM (F3,16 = 4.35, P = 0.02; Fig. 2B). CaMKII inhibition is the first kinase manipulation to prevent 3-h odor preference. Previous work showed that neither PKA (Grimes et al. 2012) nor extracellular signal-regulated kinase (ERK) (Grimes et al. 2015) is required for short-term memory. Pups in the O/O group that were infused with vehicle (41.86 ± 2.9%, n = 7) or KN-62 (42.12 ± 5.71%, n = 7) did not show a peppermint preference. Pups that received O/S and were infused with vehicle showed a peppermint preference (75.53 ± 5.42%, n = 7), while O/S pups infused with KN-62 did not, compared with O/S vehicle pups (52.47 ± 6.73%, n = 10; t = 2.9, P < 0.01). It has been shown Epac agonist bulbar infusion initiates both STM and LTM when paired with odor (Grimes et al. 2015). While Epac mediates LTM via ERK signaling, how it mediates STM is not known. This result suggests that Epac mediation of STM (Grimes et al. 2015) may operate through CaMKII. The association of Epac and CaMKII activation is well-known in cardiac models (Ruiz-Hurtado et al. 2013). This result is also consistent with evidence that AMPAR modifications occur at bulbar CaMKII sites during 3-h memory (Cui et al. 2011).
For 24-h memory (Fig. 2B), which requires activation of the cAMP/PKA/CREB pathway, CaMKII activation is also necessary. Pups in the O/O group that were infused with vehicle (38.41 ± 6.19%, n = 4) or KN-62 (36.62 ± 5.77%, n = 4) did not show a preference for peppermint. Vehicle-infused pups that received O/S training preferred peppermint (62.12 ± 7.59%, n = 6), while KN-62-infused pups receiving O/S training did not, compared with O/S vehicle pups (37.54 ± 4.21%, n = 6; t = 3.05, P < 0.01). This outcome is consistent with the reported role of CaMKII in supporting LTP and in promoting phosphorylated CREB (pCREB) activation (Ma et al. 2014).
A caveat here is that CaMKII inhibition during odor training may affect synaptic transmission and disrupt odor perception. To isolate odor memory encoding from odor perception, we further conducted experiments in which either KN-62 or the specific CaMKII inhibitory peptide tatCN21 was infused after training. One-way ANOVA revealed significant group effects (F4,20 = 25.83, P < 0.01; Fig. 2C). Either KN-62 (35.24 ± 6.14%, n = 5, t = 5.75, P < 0.01) or tatCN21 (31.33 ± 3.28%, n = 5, t = 6.5, P < 0.01) blocked odor preference learning compared with the O/S vehicle group (65.02 ± 3.10%, n = 5). The tat scrambled form had no effect on learning compared with the tatCN21 group (66.80 ± 1.45%, n = 5, t = 0.34, P = 0.73). These results suggest that the memory impairment observed with CaMKII inhibition is due to a disruption in memory encoding but not to impaired synaptic transmission or odor perception.
We hypothesized that CaMKII would have two roles in LTM. First, it would act synergistically with the cAMP pathway to promote intracellular signaling, and second, it would confer stimulus specificity. We exposed rat pups to peppermint and infused Sp-cAMPs into the OBs to directly activate PKA and induce LTM (Grimes et al. 2012). Blocking CaMKII in the presence of Sp-cAMPs permits the examination of the roles of CaMKII in the stimulus specificity of the LTM.
As reported previously (Grimes et al. 2012), Sp-cAMPs pairing with peppermint induced a preference for peppermint. A one-way ANOVA revealed significant group effects (F3,24 = 16.05, P < 0.01, Fig. 2D1). Pups had a preference for peppermint when infused with Sp-cAMPs (72.83 ± 5.24%, n = 6) compared with control pups infused with vehicle (39.35 ± 4.07%, n = 6, t = 4.85, P < 0.01). We then blocked CaMKII with KN-62 in the presence of Sp-cAMPs while exposing pups to peppermint. Pups with coinfused drugs showed a preference for peppermint (67.1 ± 4.54%, n = 8) compared with vehicle-infused pups (t = 4.27, P < 0.01). Post hoc Fisher tests show no significant difference between the KN-62+Sp-cAMPs group and the Sp-cAMPs group (t = 0.92, P > 0.05).
This result permitted us to ask whether CaMKII plays a role in odor preference specificity. We exposed rat pups to peppermint, infused with vehicle, KN-62, Sp-cAMPs, or the Sp-cAMPs+KN-62 mixture and then tested for preferences for a control odor, vanillin. A one-way ANOVA demonstrated significant group effects (F3,21 = 6.42, P < 0.01, Fig. 2D2). Rat pups receiving Sp-cAMPs (34.46 ± 5.74%, n = 6), KN-62 (34.55 ± 1.91%, n = 5), or vehicle (46.49 ± 8.11%, n = 6) showed no preference for vanillin. However, rat pups in the Sp-cAMPs+KN-62 group showed a significant preference for vanillin (68.26 ± 7%, n = 8) compared with pups in the vehicle group (t = 2.43, P < 0.05), the KN-62 group (t = 3.56, P < 0.01), or the Sp-cAMPs group (t = 3.77, P < 0.01). This demonstrates that PKA activation paired with odor creates an odor-specific LTM but coinfusing a CaMKII antagonist disrupts odor specificity and causes a generalized approach to novel odors, consistent with CaMKII operating as a synaptic tag.
Stimulus specificity of odor preference depends on CaMKII-mediated AMPAR insertion.
AMPAR insertion has been shown to support LTM in early odor preference learning (Cui et al. 2011), as in other models (Kessels and Malinow 2009). In our third set of experiments we assessed AMPAR insertion in odor-specific preference and generalized odor preference.
O/S training increased GluA1 synaptic expression, which was blocked in the presence of KN-62. A one-way ANOVA showed significant group effects (F2,25 = 3.73, P < 0.05; Fig. 3A). Rat pups infused with vehicle in the O/S group (1.92 ± 0.22, n = 11) had more GluA1 expression than pups infused with KN-62 (1.13 ± 0.28, n = 6, t = 2.56, P < 0.05). These findings suggest that functional CaMKII is critical for normal AMPAR insertion with learning. In another set of experiments pups were infused with Sp-cAMPs or the drug cocktail Sp-cAMPs+KN-62. There is again a significant group effect (F2,21 = 5.04, P < 0.05; Fig. 3B). Pups in the Sp-cAMPs group (2.46 ± 0.37, n = 7) showed higher levels of GluA1 than pups in the vehicle (1.42 ± 0.13, n = 11, t = 0.76, P < 0.05) or Sp-cAMPs+KN-62 (1.26 ± 0.37, n = 7, t = 2.90, P < 0.01; Fig. 3B) condition. The loss of stimulus specificity was associated with a loss of AMPAR increases.
Fig. 3.
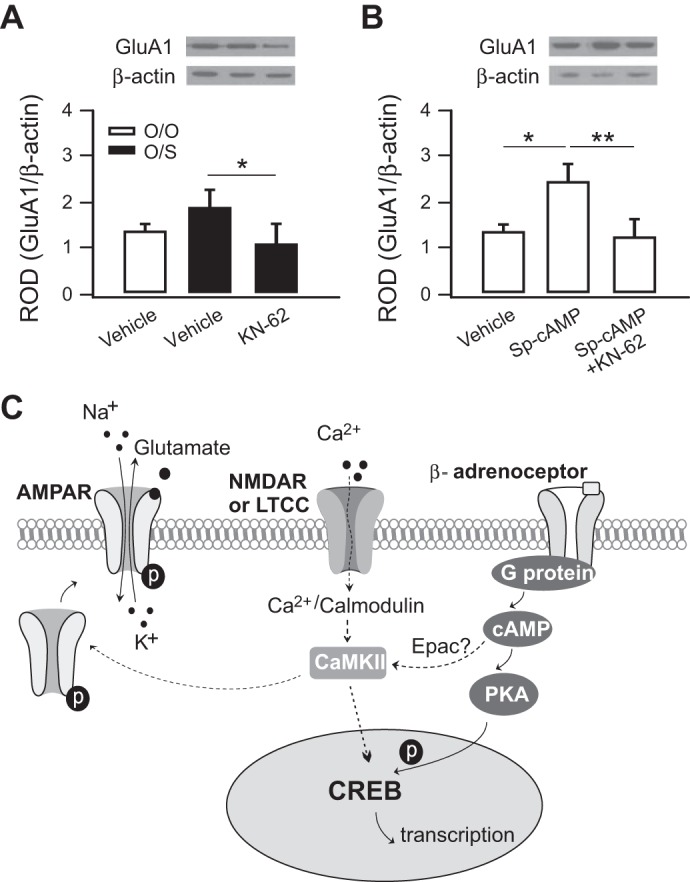
Roles of CaMKII and PKA in AMPA receptor (AMPAR) synaptic insertion. A: relative optical density (ROD) of AMPAR GluA1 subunits over β-actin in KN-62-infused and control pups. O/O, odor only; O/S, odor+stroking. *P < 0.05. B: ROD of GluA1 over β-actin in Sp-cAMPs-, Sp-cAMPs+KN-62-, or vehicle-infused pups. **P < 0.01. C: schematics of the interactions of CaMKII and cAMP/PKA in promoting AMPAR insertion and CREB-mediated transcription. We propose that odor-evoked calcium entry through NMDAR or L-type calcium channel (LTCC) converges with β-adrenoceptor-mediated cAMP/Epac pathway to activate CaMKII, which in turn phosphorylates AMPAR and promotes AMPAR insertion into the synaptic membrane. The enhanced AMPAR insertion serves as a mechanism for short-term memory as well as a synaptic tag for long-term memory. CaMKII also interacts with PKA signaling to promote CREB phosphorylation and gene transcription, which is necessary for long-term memory.
DISCUSSION
Increased CaMKII phosphorylation was observed in the dorsolateral region of the OB after early odor preference learning, consistent with the “hot spot” following peppermint conditioning reported previously with 2-deoxy-[14C]glucose (Woo and Leon 1987) and pCREB (McLean et al. 1999). At which synapses in the OB does the CaMKII activation occur after the odor-specific preference learning? The increase in pCaMKII was seen in both the glomerular layer and the external plexiform layer. The likely scenario is that the transmission between the ON and the MC synapse is enhanced during learning and this activates CaMKII in MC dendritic synapses in the glomerulus receiving the ON input. Enhanced MC activities promote increased inhibition from connected granule cells and therefore increase CaMKII phosphorylation at the MC-to-granule cell synapses at the external plexiform layer. This pattern is consistent with enhanced expression of the immediate-early gene Arc in both mitral and granule cells after early odor preference learning (Shakhawat et al. 2014).
The phosphorylation of CaMKII T286 promotes its translocation to the PSD, where it is trapped for several minutes (Shen et al. 2000) and can regulate AMPAR by membrane insertion (Hayashi et al. 2000). A second autophosphorylation (T305) dissociates CaMKII from the PSD into cytosol, preventing protease degradation (Shen et al. 2000). Dissociated CaMKII remains phosphorylated at T286 for some time and has priming effects on subsequent stimulation. We did not examine cytosol changes but focused on changes in synaptic membrane.
The present results show that disrupting CaMKII prevents acquisition of naturally induced odor preference STM and LTM. The data suggest that odor preference STM may be supported by Epac recruitment of CaMKII (Grimes et al. 2015) and stimulus-specific enhanced AMPAR function. Epac activation leads to 3-h odor preference memory (Grimes et al. 2015), and CaMKII inhibitor blocks 3-h memory (present data); therefore we propose a likely interaction of the two since other candidates such as PKA or ERK blockade do not affect 3-h memory (Grimes et al. 2013, 2015). For STM CaMKII could enhance specific synapses through enhancement of AMPAR conductance and promotion of AMPAR insertion through the phosphorylation of stargazin (Boehm and Malinow 2005). CaMKII synergizes with the PKA cascade to support LTM, but direct and strong PKA activation, which induces odor-specific preference with CaMKII, results in generalized novel odor approach without CaMKII. These outcomes are consistent with CaMKII synaptic tagging-mediation of stimulus specificity via AMPAR insertion (Fig. 3C).
Besides MCs, CaMKII is also expressed in the olfactory receptor neuron in rat pups, as in mice (Wei et al. 1998). Odor binding to the olfactory receptor activates cAMP signaling and leads to the opening of cyclic nucleotide-gated channels (Reed 1992). Calcium entry through these channels activates CaMKII, which attenuates cAMP and leads to the termination of the receptor signaling (Wei et al. 1998). Therefore a caveat is that signal transduction and receptor excitation is prolonged in the presence of either CaMKII blockade or cAMP activation and this subsequently alters odor perception. It is unlikely, however, that odor perception was disrupted with these drug infusions since Sp-cAMPs-infused pups showed normal patterns of learning and pups that had infusions of CaMKII inhibitors immediately after training showed impaired odor preference learning. In the latter case, memory encoding was disrupted but not odor perception.
AMPAR increases are not seen with the nonspecific odor-approach response produced by the drug cocktail. This is consistent with mediation of AMPAR insertion by CaMKII. What mediates generalized preference is not clear to us. Although this is a novel finding in preference learning, there are data on generalized avoidance. Intrabulbar disinhibition via bicuculline produces generalized odor avoidance in PND12 pups (Okutani et al. 2002). These effects were related to excitability increases, and such increases were proposed to couple odors nonspecifically to the avoidance behaviors that are readily acquired at PND12.
More recently, activation of cAMP/PKA signaling has been shown to increase neuronal excitability in the amygdala and induce generalized fear to nonconditioned stimuli (Ghosh and Chattarji 2015). PKA activation has also been shown to induce long-term increases in neuronal excitability in neonate rats in other brain neurons and does not require CaMKII (Bakhshishayan et al. 2013). Thus, in the present study, one possibility is that increased MC excitability mediates generalized odor preference behavior at PND7, but if CaMKII is intact a stimulus-specific memory induced by synaptic plasticity overrides or prevents generalized approach responses.
GRANTS
This work was supported by a Canadian Institutes of Health Research (CIHR) operating grant (MOP-102624) and a Memorial University Dean's transition grant to Q. Yuan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.M., B.M., J.H.M., C.W.H., and Q.Y. conception and design of research; S.M., B.M., and Q.Y. performed experiments; S.M., B.M., and Q.Y. analyzed data; S.M., B.M., C.W.H., and Q.Y. interpreted results of experiments; S.M., B.M., and Q.Y. prepared figures; S.M., B.M., C.W.H., and Q.Y. drafted manuscript; S.M., B.M., J.H.M., C.W.H., and Q.Y. approved final version of manuscript; J.H.M., C.W.H., and Q.Y. edited and revised manuscript.
REFERENCES
- Bakhshishayan S, Enomoto A, Tsuji T, Tanaka S, Yamanishi T, Ishihama K, Kogo M. Protein kinase A regulates the long-term potentiation of intrinsic excitability in neonatal trigeminal motoneurons. Brain Res 1541: 1–8, 2013. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993. [DOI] [PubMed] [Google Scholar]
- Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem Soc Trans 33: 1354–1356, 2005. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Taylor D. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol 55: 696–711, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Darby-King A, Grimes MT, Howland JG, Wang YT, McLean JH, Harley CW. Odor preference learning and memory modify GluA1 phosphorylation and GluA1 distribution in the neonate rat olfactory bulb: testing the AMPA receptor hypothesis in an appetitive learning model. Learn Mem 18: 283–291, 2011. [DOI] [PubMed] [Google Scholar]
- Fontaine CJ, Mukherjee B, Morrison GL, Yuan Q. A lateralized odor learning model in neonatal rats for dissecting neural circuitry underpinning memory formation. J Vis Exp 90: e51808, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature 385: 533–536, 1997. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci 18: 112–120, 2015. [DOI] [PubMed] [Google Scholar]
- Grimes MT, Harley CW, Darby-King A, McLean JH. PKA increases in the olfactory bulb act as unconditioned stimuli and provide evidence for parallel memory systems: pairing odor with increased PKA creates intermediate- and long-term, but not short-term, memories. Learn Mem 19: 107–115, 2012. [DOI] [PubMed] [Google Scholar]
- Grimes MT, Powell M, Gutierrez SM, Darby-King A, Harley CW, McLean JH. Epac activation initiates associative odor preference memories in the rat pup. Learn Mem 22: 74–82, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes MT, Smith M, Li X, Darby-King A, Harley CW, McLean JH. Mammalian intermediate-term memory: new findings in neonate rat. Neurobiol Learn Mem 95: 385–391, 2011. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267, 2000. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron 61: 340–350, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethbridge R, Hou Q, Harley CW, Yuan Q. Olfactory bulb glomerular NMDA receptors mediate olfactory nerve potentiation and odor preference learning in the neonate rat. PLoS One 7: e35024, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu Y, Zhong P, Wilkinson B, Qi J, Olsen CM, Bayer KU, Liu QS. CaMKII activity in the ventral tegmental area gates cocaine-induced synaptic plasticity in the nucleus accumbens. Neuropsychopharmacology 39: 989–999, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, Zhang G, Neubert TA, Tsien RW. GammaCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159: 281–294, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature 340: 554–557, 1989. [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW, Darby-King A, Yuan Q. pCREB in the neonate rat olfactory bulb is selectively and transiently increased by odor preference-conditioned training. Learn Mem 6: 608–618, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee B, Harley CW, Yuan Q. Learning-induced metaplasticity? Associative training for early odor preference learning down-regulates synapse-specific NMDA receptors via mGluR and calcineurin activation. Cereb Cortex (October 26, 2015). doi: 10.1093/cercor/bhv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okutani F, Zhang JJ, Yagi F, Kaba H. Non-specific olfactory aversion induced by intrabulbar infusion of the GABAA receptor antagonist bicuculline in young rats. Neuroscience 112: 901–906, 2002. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Perlman S, Malinow R. Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science 266: 1881–1885, 1994. [DOI] [PubMed] [Google Scholar]
- Raineki C, Pickenhagen A, Roth TL, Babstock DM, McLean JH, Harley CW, Lucion AB, Sullivan RM. The neurobiology of infant maternal odor learning. Braz J Med Biol Res 43: 914–919, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RR. Signaling pathways in odorant detection. Neuron 8: 205–209, 1992. [DOI] [PubMed] [Google Scholar]
- Ruiz-Hurtado G, Morel E, Dominguez-Rodriguez A, Llach A, Lezoualc'h F, Benitah JP, Gomez AM. Epac in cardiac calcium signaling. J Mol Cell Cardiol 58: 162–171, 2013. [DOI] [PubMed] [Google Scholar]
- Sanhueza M, Lisman J. The CaMKII/NMDAR complex as a molecular memory. Mol Brain 6: 10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhawat AM, Gheidi A, Hou Q, Dhillon SK, Marrone DF, Harley CW, Yuan Q. Visualizing the engram: learning stabilizes odor representations in the olfactory network. J Neurosci 34: 15394–15401, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci 3: 881–886, 2000. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science 257: 206–211, 1992a. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science 257: 201–206, 1992b. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci 114: 957–962, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience 144: 825–833, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villers A, Giese KP, Ris L. Long-term potentiation can be induced in the CA1 region of hippocampus in the absence of alphaCaMKII T286-autophosphorylation. Learn Mem 21: 616–626, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Zhao AZ, Chan GC, Baker LP, Impey S, Beavo JA, Storm DR. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in neurons: a mechanism for attenuation of olfactory signals. Neuron 21: 495–504, 1998. [DOI] [PubMed] [Google Scholar]
- Woo CC, Leon M. Sensitive period for neural and behavioral response development to learned odors. Brain Res 433: 309–313, 1987. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH. Mitral cell beta1 and 5-HT2A receptor colocalization and cAMP coregulation: a new model of norepinephrine-induced learning in the olfactory bulb. Learn Mem 10: 5–15, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Shakhawat AM, Harley CW. Mechanisms underlying early odor preference learning in rats. Prog Brain Res 208: 115–156, 2014. [DOI] [PubMed] [Google Scholar]