Figure 6. C9orf72 interacts with Rab1a.
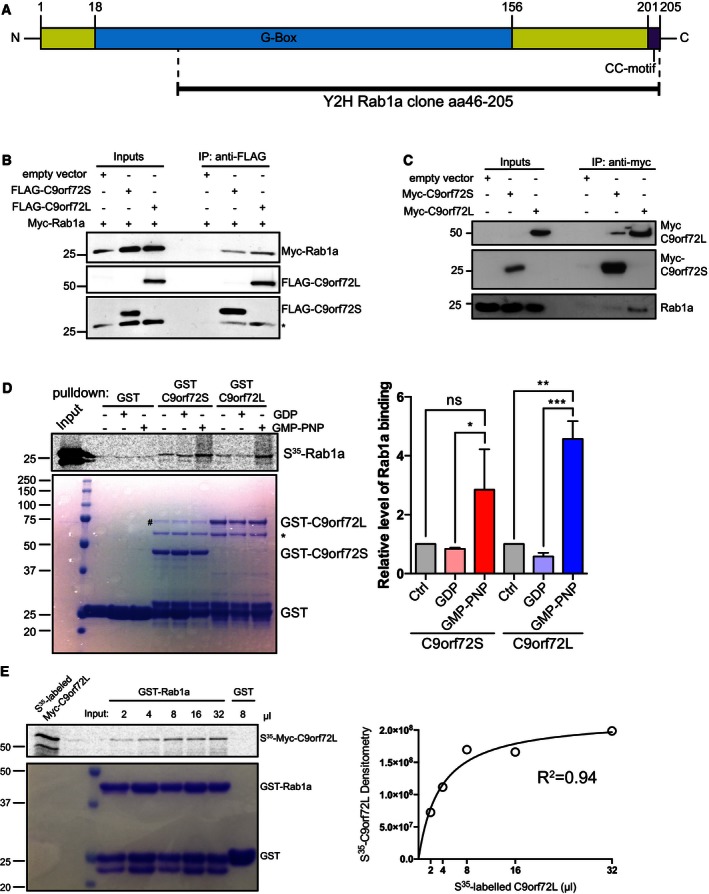
- A human brain random cDNA library was screened in a Y2H assay using C9orf72S as bait. A clone coding for aa 46–205 of Rab1a, comprising most of the GTP‐binding G‐box domain and the C‐terminal CC‐motif was found to interact with C9orf72S.
- Cell lysates of HEK293 cells co‐transfected with Myc‐Rab1a and either empty vector, FLAG‐C9orf72S, or FLAG‐C9orf72L were subjected to immunoprecipitation with anti‐FLAG antibody. Bound protein was eluted from beads using excess FLAG peptide. Immune eluates were probed for FLAG‐C9orf72 and Myc‐Rab1a on immunoblots. The input levels of FLAG‐C9orf72 and Myc‐Rab1a in the transfected cells are shown (Inputs). * indicates remaining Myc‐Rab1a signal after reprobing for FLAG‐C9orf72.
- Cell lysates of HEK293 cells transfected with Myc‐C9orf72S or Myc‐C9orf72L were subjected to immunoprecipitation with anti‐Myc antibody. The resulting immune pellet was probed for endogenous Rab1a.
- 35S‐radiolabeled recombinant Myc‐Rab1a protein loaded with vehicle, GDP or GMP‐PNP was added to GST, GST‐C9orf72S, and GST‐C9orf72L immobilized on glutathione‐coated beads. 35S‐radiolabeled recombinant Myc‐Rab1a protein was visualized by phosphorimager (top panel). Coomassie‐stained GST, GST‐C9orf72S, and GST‐C9orf72L in the pull‐down samples are shown (bottom panel). The identity of the Coomassie protein bands was confirmed by mass spectrometry (# indicates E. coli DnaK chaperonin; * indicates E. coli 60kD chaperonin; Appendix Fig S3). Relative binding of Rab1a to C9orf72 was quantified from 3 independent experiments (mean ± SEM; one‐way ANOVA with Fisher's LSD test; ns, not significant; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
- Increasing volumes of 35S‐radiolabeled recombinant Myc‐C9orf72L protein were incubated with equal amounts of GST‐Rab1a immobilized on glutathione‐coated beads in an equilibrium binding experiment. 8 μl of 35S‐radiolabeled recombinant Myc‐C9orf72L protein was incubated with GST as a negative control. Bound 35S‐radiolabeled Myc‐C9orf72L protein was visualized by phosphorimager. Coomassie‐stained GST‐Rab1a and GST in the pull‐down samples are shown. Densitometry analysis of the amount of 35S‐radiolabeled recombinant Myc‐C9orf72L protein bound to GST‐Rab1a in the different binding reactions was used to fit an equilibrium binding hyperbola (R 2 = 0.94).
Source data are available online for this figure.
