Figure EV1. Removal of the C‐terminal moiety of TssA1 leads to its monomeric form.
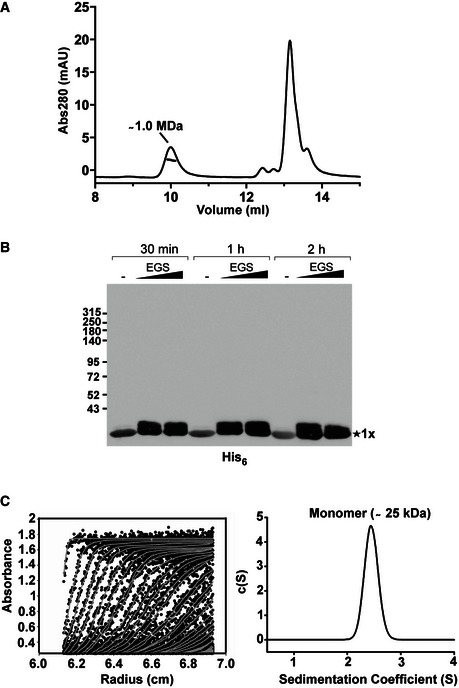
- SEC‐MALS analysis of the purified His6‐TssA1. Experiments were carried out on an Agilent Technologies 1200 Series HPLC using a WYATT 100S5 column coupled to a WYATT MALS instrument. The eluted peaks of His6‐TssA1 and the corresponding mass are shown.
- Western blot showing in vitro cross‐linking experiments using the purified C‐terminally truncated protein TssA11–245. About 30 μg of purified His6‐TssA11–245 was cross‐linked (30 min, 1 and 2 h) at room temperature using increasing amounts of EGS (2 and 5 mM) where indicated. Samples were analysed by 3–12% gradient SDS–PAGE and TssA11–245 immunodetected using an anti‐His6 monoclonal antibody.
- Sedimentation data of His6‐TssA11–245 (1.6 mg/ml) recorded at a rotor speed of 50,000 rpm are shown. The left panel shows the sedimentation boundary fits. For clarity, only every third scan is shown in the fitted data plots where the experimental absorbance data are shown as black circles, whereas the boundary fits are shown as grey lines. The right panel shows the size‐distribution analysis c(s), obtained from fitting the scan boundaries using SEDFIT, revealing a monomeric peak (Mr ˜25 kDa) at S20,w value of 2.4 S.
