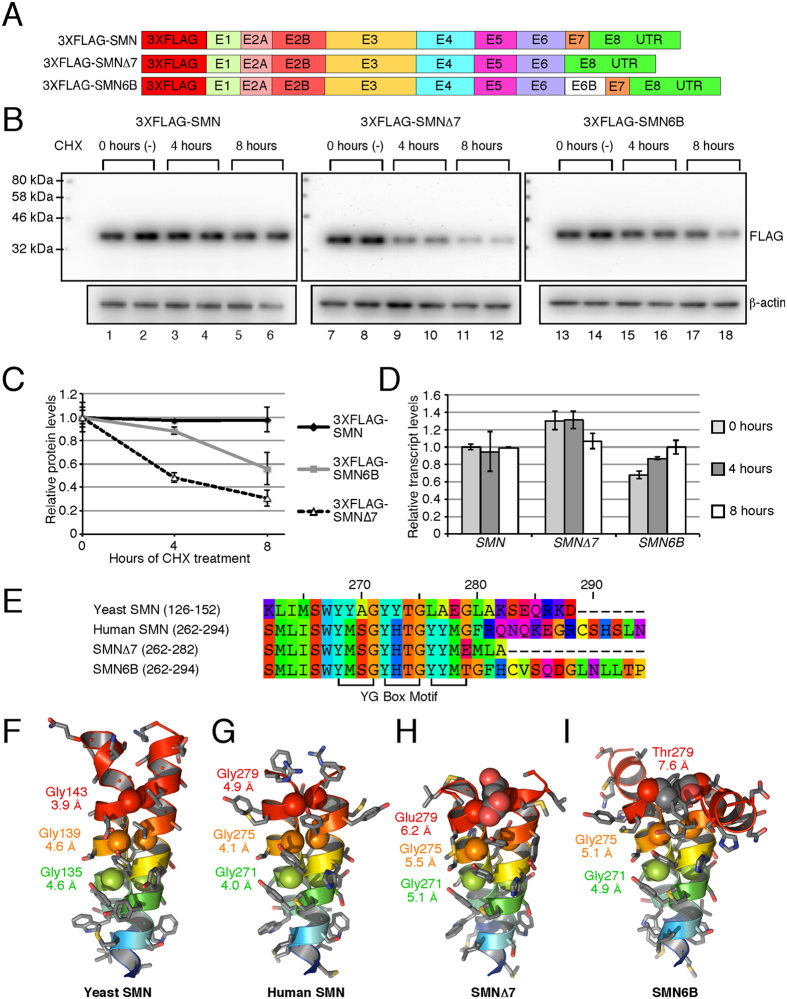
Figure 5. Stability of SMN6B protein.
(A) Diagrammatic representation of the cDNA constructs used for the determination of protein stability. (B) Western blots showing the effect of 20 μg/ml CHX treatment on the level of 3XFLAG-tagged proteins. 12 μg of total protein was loaded in each lane. (C) Densitometric quantification of Western blots shown in (B). Values are expressed as relative to the average value at 0 hours. Error bars represent standard error. (D) QPCR of transfected SMN after CHX treatment. We generated cDNA employing 3′UTR primer. QPCR were performed using primers 5′FLAG (anneals within 3XFLAG) and 3′E1+2A (anneals exon 1/exon 2A junction). All values are expressed as relative to untreated SMN and normalized by neomycin resistance gene on the same transfected plasmid using primers 5′NEO (anneals within neomycin) and 3′RT-Univ (anneals 3′UTR adapter). Error bars represent standard error. (E) The sequence alignment compares the YG Box domains of Schizosaccharomyces pombe (yeast) SMN (UniProtKB accession: Q09808), human SMN (UniProtKB accession: Q16637-1), SMN∆7 (UniProtKB accession: Q16637-3) and SMN6B proteins. Residue reference numbers at the top are based on the human SMN. The positions of the conserved YG Box motif comprised of the (YXXG)3 tetrad repeat sequence is indicated at the bottom. (F,G) The YG Box domains mediate dimerization through a coiled-coil interaction observed in the crystal structure of the yeast SMN (PDB code: 4RG5) and human SMN (PDB code: 4GLI) proteins. (H,I) Symmetric homology modeling of the YG Box domains from the SMN∆7 and SMN6B isoforms shows the potential for steric distortion in the dimeric structures due to substitution of the last conserved residue of the YG Box motif, Gly279. Structure and models are shown in cartoon representations with a blue to red rainbow color scheme from N- to C-terminus. The last six residues in the SMN6B model are unstructured and not shown for clarity. Side chains are shown as stick representations with coloring by element: grey for carbon, red for oxygen, blue for nitrogen, and gold for sulfur. The Cα atoms of the conserved glycine residues are shown as van der Waals spheres. The inter-atomic distances between Cα atoms of the symmetry related glycine residues are indicated below the labels for each residue.