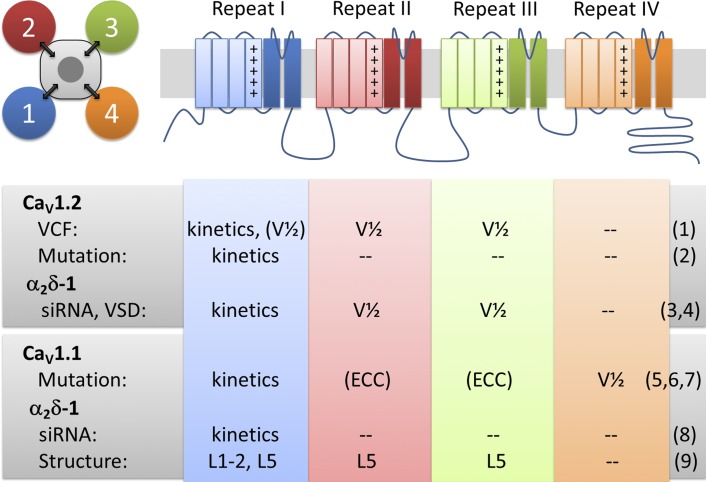
Figure 1.
Pseudotetrameric domain structure of CaVs with functions associated to each VSD. Each of the four homologous repeats contains a functional VSD (first four transmembrane helices) and contributes one fourth of the channel pore. Voltage-clamp fluorometry (VCF), mutagenesis, siRNA knockdown, and structure studies provide evidence for distinct functions and interactions of each of the four VSDs of CaV1.2 and CaV1.1 channels and their modulation by α2δ-1. Kinetics and V1/2 refer to kinetics and voltage dependence of activation, respectively. L1-2 and L5 refer to extracellular loops connecting transmembrane helices S1 and S2 and helix S5 with the pore, respectively. References: (1) Pantazis et al. (2014); (2) Nakai et al. (1994); (3) Tuluc et al. (2007); (4) Savalli et al. (2016) in this issue; (5) Tuluc et al. (2016a); (6) Eltit et al. (2012); (7) Tuluc et al. (2009); (8) Obermair et al. (2005); (9) Wu et al. (2015).