Abstract
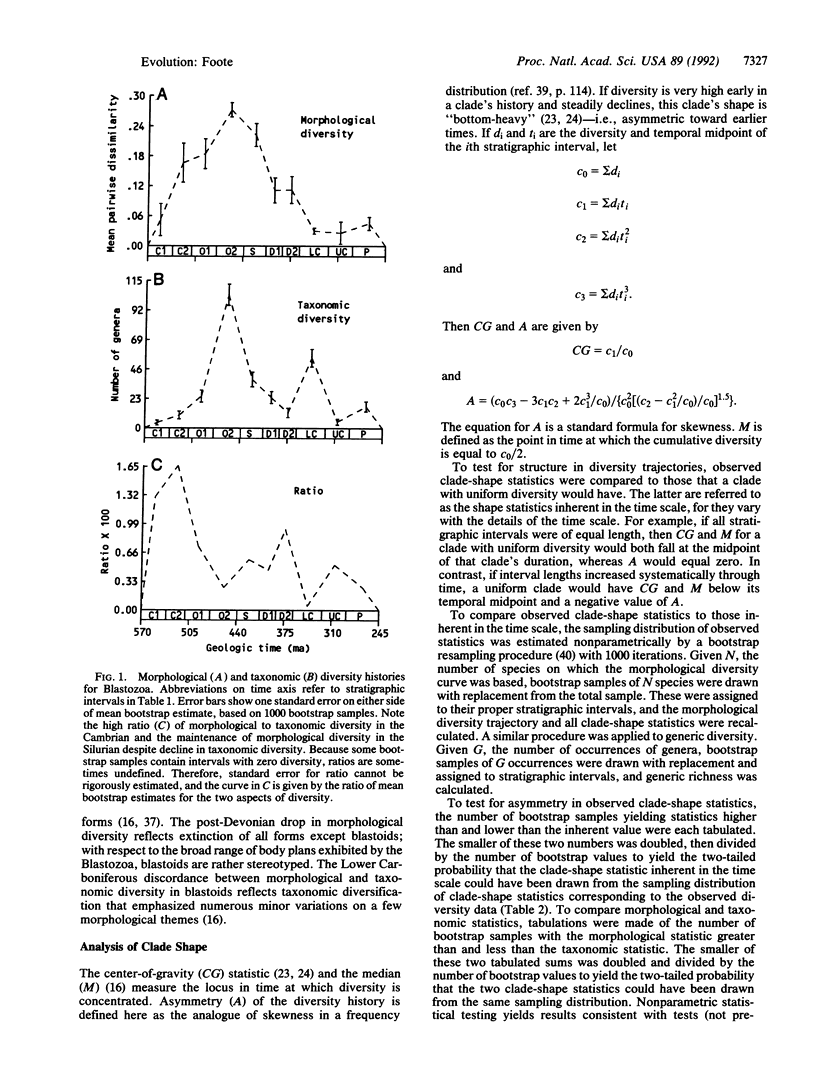
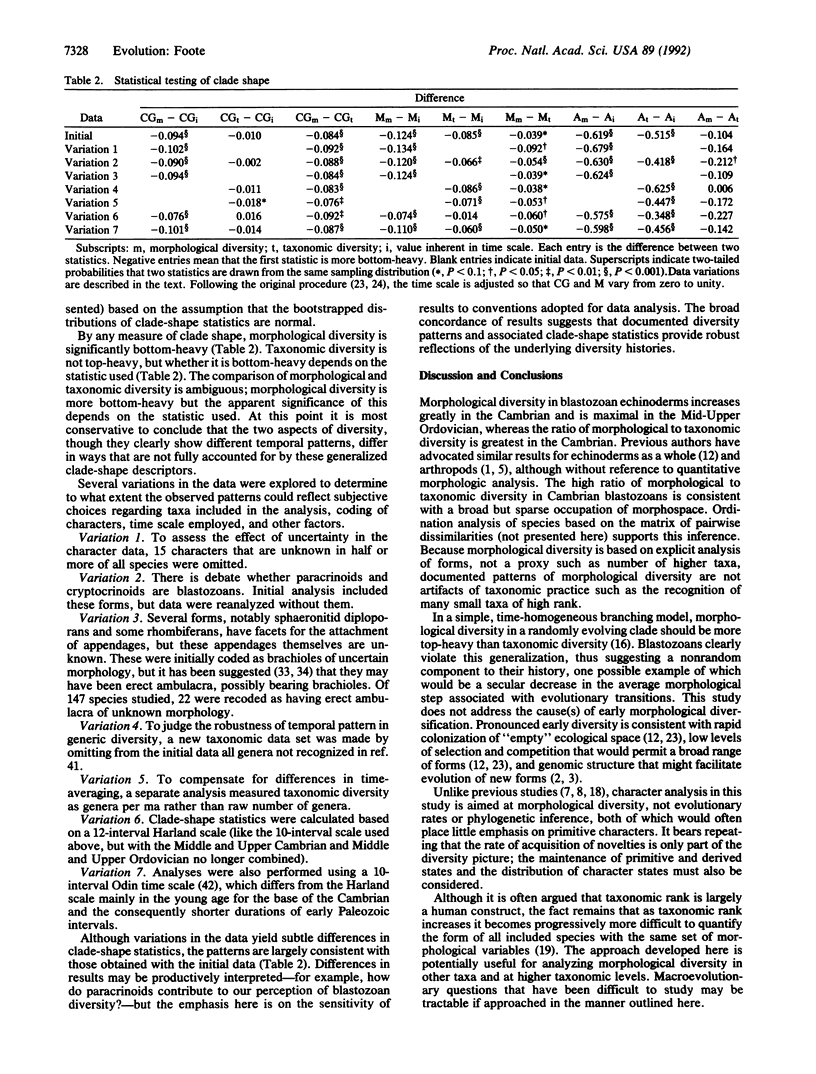
There has been extensive debate about the magnitude and implications of morphological diversity in early Paleozoic animals, with some workers using apparently rapid initial diversification to infer unusual evolutionary processes. Analysis of discrete morphological characters shows that initial morphological diversification in the echinoderm subphylum Blastozoa was so pronounced that morphological diversity relative to taxonomic diversity was greatest in the Cambrian, whereas morphological diversity itself was greatest in the Middle and Upper Ordovician. Thus, a small number of Cambrian taxa sparsely occupied a large range in morphological space, whereas subsequent diversification involved expansion and filling of morphospace. A measure of clade-shape asymmetry and a method for statistical testing of clade shape are used to show that morphological diversity is significantly concentrated early in the history of the Blastozoa. The subphylum represents the highest biologic level at which temporal patterns of morphological diversity have been analyzed. Because this study is based on explicit morphological analysis, not taxonomic proxies for morphological diversity, the results are not artifacts of taxonomic practice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs D. E., Fortey R. A. The early radiation and relationships of the major arthropod groups. Science. 1989 Oct 13;246(4927):241–243. doi: 10.1126/science.246.4927.241. [DOI] [PubMed] [Google Scholar]
- Erwin D. H., Valentine J. W. "Hopeful monsters," transposons, and Metazoan radiation. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5482–5483. doi: 10.1073/pnas.81.17.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Gilinsky N. L., German R. Z. Asymmetry of lineages and the direction of evolutionary time. Science. 1987 Jun 12;236(4807):1437–1441. doi: 10.1126/science.236.4807.1437. [DOI] [PubMed] [Google Scholar]
- Jacobs D. K. Selector genes and the Cambrian radiation of Bilateria. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4406–4410. doi: 10.1073/pnas.87.11.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]


