Figure 3.
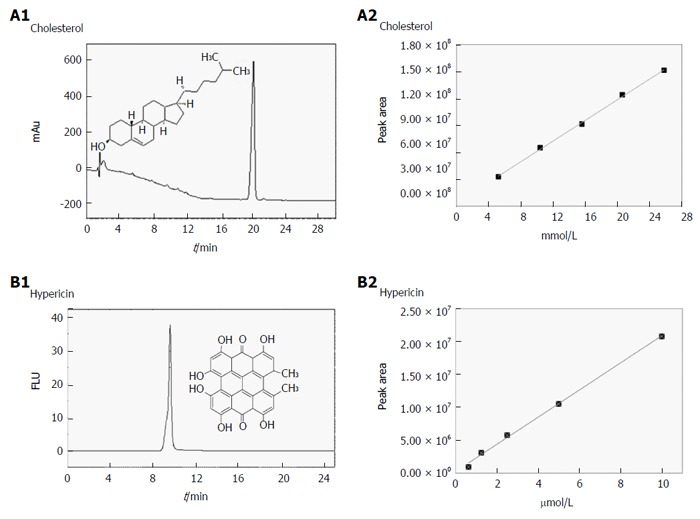
Validation of the chromatographic methods for cholesterol and hypericin quantification in gallstones. A1-B2: Validation data of the analysis by reverse phase-high performance liquid chromatography on hypericin pre-incubated human gallstones with UV (204 nm) and fluorescence detection (excitation/emission wavelengths: 470/600 nm). A typical UV chromatogram of 5.2 × 10-3 M cholesterol with a retention time (RT) of 19 ± 0.22 min (A1) and the HPLC-generated calibration curve (A2) based on four replicate measurements of five working solutions of cholesterol are presented. A representative fluorescence chromatogram of 1.3 × 10-6 M hypericin with an RT of 9.68 ± 0.06 min (B1) along with corresponding HPLC-generated calibration curve of hypericin (B2) is shown. FLU: Fluorescence units; mAu: Milli-absorbance units.
