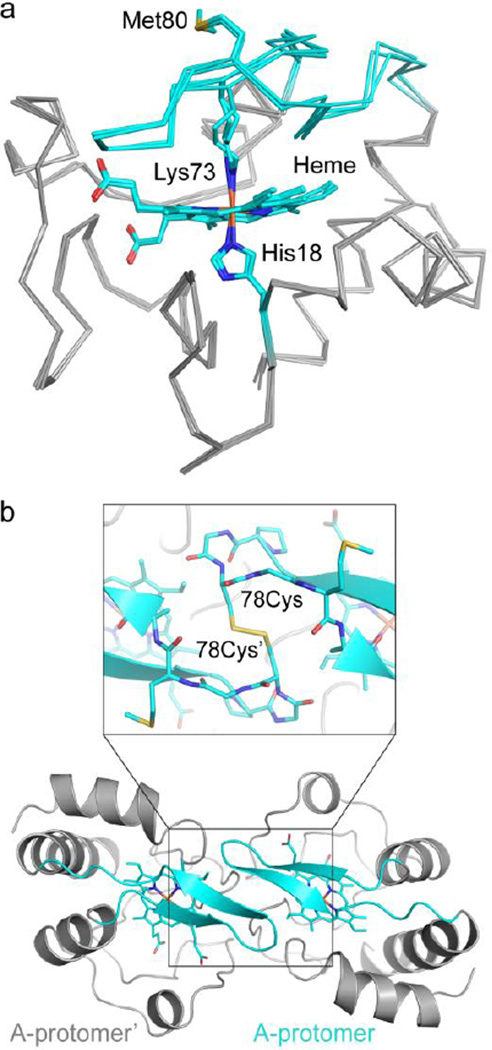
Figure 2.
The T78C/K79G crystal structure reveals Lys73-coordination to the heme iron. (a) Alignment of the three molecules in the asymmetric unit of T78C/K79G cyt c (gray Cα trace, with cyan coordination loop) by their main chain atoms reveals a similar overall structure, RMSD < 0.3 Å. In all three molecules, the heme iron is coordinated by His18 and Lys73. Met80 is solvent-exposed, as labeled. Heme, His18, Lys73, and Met80 are shown as stick figures, with cyan carbons and non-carbon atoms colored by element: N=blue, O=red, S=yellow, Fe=orange. (b) Each protomer of the asymmetric unit forms a dimer with a molecule related by a two-fold symmetry axis. Here, chain A is shown with respect to its symmetry mate, chain A’ (both in gray cartoon, with coordination loop colored cyan), and the covalent dimer interaction is highlighted. Inset: the Cys78 residue forms a disulfide bond with Cys78’ (proteins in cartoon and stick representation). Figure S5c shows non-covalent interactions at the dimer interface.