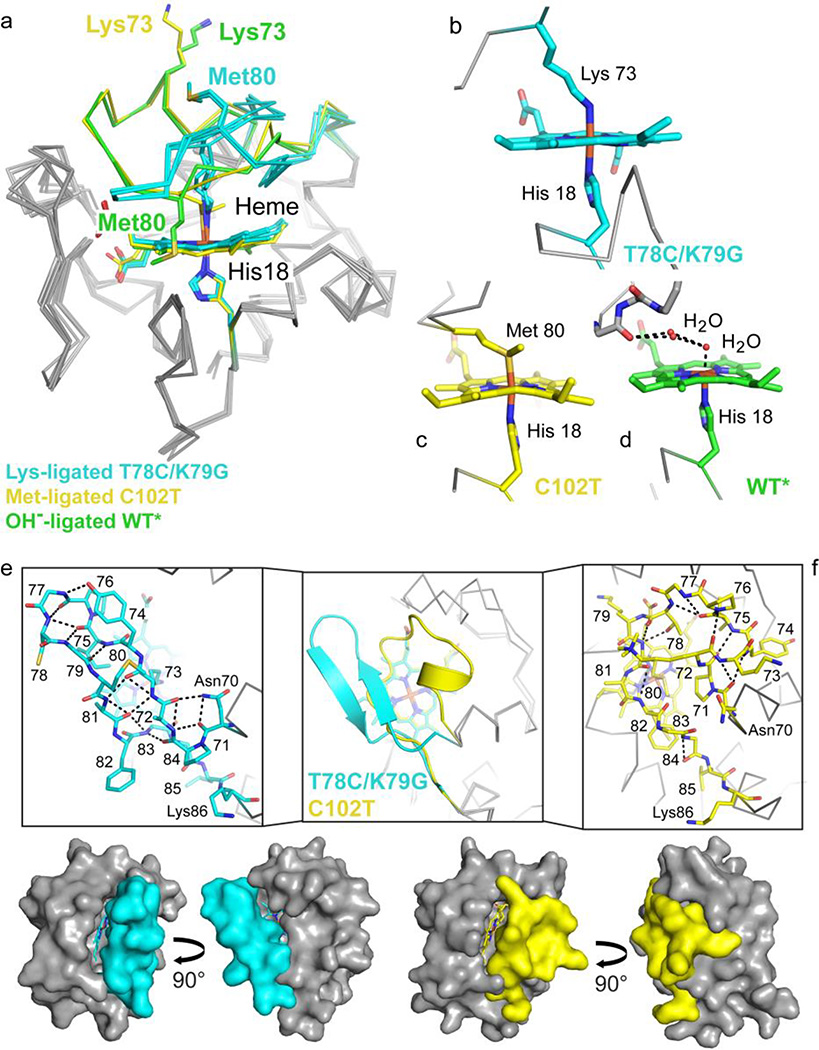
Figure 7.
Coordination loop differences in cyt c structures with Lys73, hydroxide (PDB ID: 4MU8),29 or Met80 (PDB ID: 2YCC)25 coordinated to the heme iron. Structures are colored by carbon atoms as follows: Lys73-ligated T78C/K79G=cyan, Met80-ligated C102T=yellow, hydroxide-ligated WT*=green. Non-carbon atoms are colored by element: N=blue, O=red, S=yellow, Fe=orange. (a) Alignment of the three structures by main-chain atoms reveal similar core conformations, with RMSD = 0.50 Å and 0.27/0.33 Å (A- and B-protomers), respectively. The Lys73 and Met80 residues are highlighted (side chain stick figures) and labeled for each structure. (b–d) Heme coordination geometry for Lys73-ligated T78C/K79G (b), Met80-ligated C102T (c), and WT* (d), as labeled. (e–f) The heme coordination loops of T78C/K79G (e) and hydroxide-ligated C102T (f) are shown following superposition of the core conformations in cartoon representation (middle box) and as stick figures (left and right boxes). Stick representations of the loops are labeled by residue, and black dashed lines mark hydrogen bonds. Below, the van der Waals surfaces for T78C/K79G (cyan) and C102T (yellow) are shown to highlight the positions of the heme coordination loop.