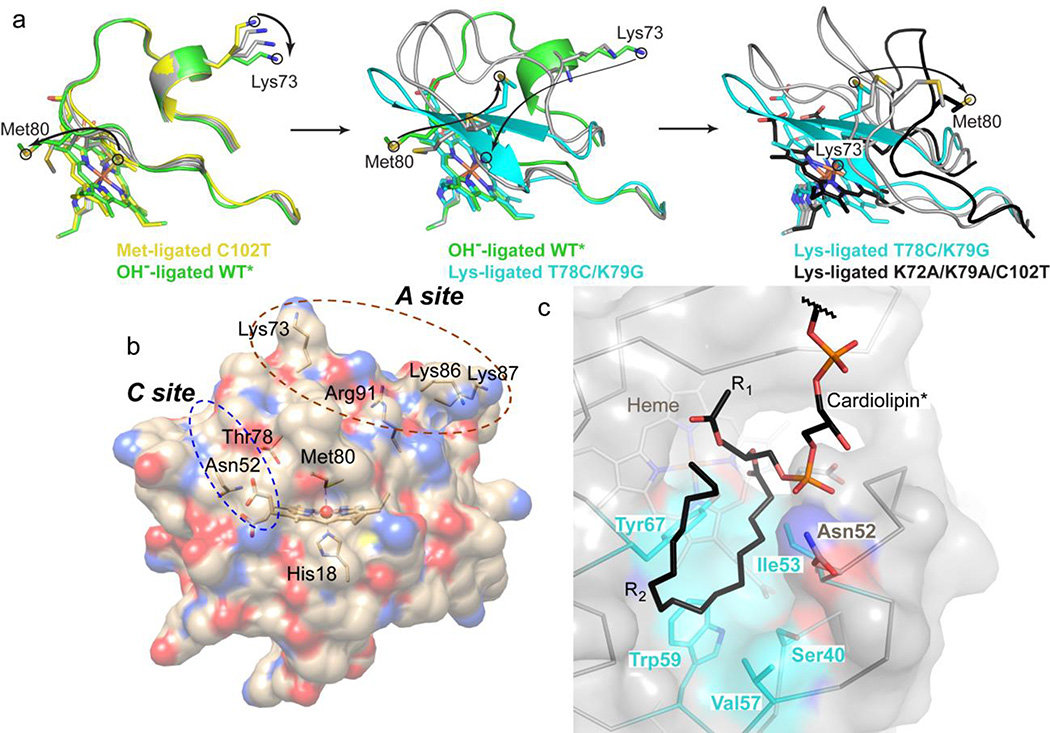
Figure 9.
(a) Model of the Met80-to-Lys73 structural transition in cyt c. To aid in visualization of the transition, “intermediate” states were generated in PyMol using the Met80-ligated C102T25 (yellow), hydroxide-ligated WT*29 (green), Lys73-ligated T78C/K79G (cyan) and Lys73-ligated K72A/K79A/C102T30 (black) structures. Each panel (Met80-to-hydroxide, left; hydroxide-to-Lys73, middle; and further unfolding of the Lys73-ligated conformer, right) contains two “intermediate” states generated by morphing (gray carbons). Arrows indicate movements of Met80 and Lys73 to accommodate the change. (b) Structure of cyt c showing the proposed CL binding sites. At the hydrophobic C site, hydrogen bonding of the CL phospholipid group to Asn52 has been suggested to aid in the acyl insertion into the protein interior.96 (c) Structural model of CL binding into the identified hydrophobic pocket created using Coot105 and based on the positive electron density in the Fo−Fc map (Figure S16c).