Abstract
Inflammation in arterial walls leads to coronary artery disease (CAD). Because specialized proresolving lipid mediators (SPMs; lipoxins, resolvins, and protectins) stimulate resolution of inflammation in animal models, we tested whether n-3 fatty acids impact SPM profiles in patients with CAD and promote clot remodeling. Six patients with stable CAD were randomly assigned to either treatment with daily 3.36 g Lovaza for 1 yr or without. Targeted lipid mediator–metabololipidomics showed that both groups had absence of resolvin D1 (RvD1), RvD2, RvD3, RvD5 and resolvin E1—all of which are present in healthy patients. Those not taking Lovaza had an absence of aspirin-triggered resolvin D3 (AT-RvD3) and aspirin-triggered lipoxin B4 (AT-LXB4). Lovaza treatment restored AT-RvD3 and AT-LXB4 and gave levels of RvD6 and aspirin-triggered protectin D1 (AT-PD1) twice as high (resolvin E2 ∼5 fold) as well as lower prostaglandins. Principal component analysis indicated positive relationships for patients with CAD who were receiving Lovaza with increased AT-RvD3, RvD6, AT-PD1, and AT-LXB4. SPMs identified in Lovaza-treated patients with CAD enhanced ∼50% at 1 nM macrophage uptake of blood clots. These results indicate that patients with CAD have lower levels and/or absence of specific SPMs that were restored with Lovaza; these SPMs promote macrophage phagocytosis of blood clots. Together, they suggest that low vascular SPMs may enable progression of chronic vascular inflammation predisposing to coronary atherosclerosis and to thrombosis.—Elajami, T. K., Colas, R. A., Dalli, J., Chiang, N., Serhan, C. N., Welty, F. K. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling.
Keywords: metabololipidomics, resolvins, lipoxins, n-3 fatty acids, inflammation
Cardiovascular diseases (CVDs) are the leading cause of death in the world and, in 2012, accounted for 17.5 million deaths (1). It is now widely appreciated that atherosclerosis is a disease of chronic inflammation in the arterial wall that is characterized by up-regulation of vascular cell adhesion molecules, monocyte infiltration, and differentiation to macrophages in the subendothelial space leading to foam cells [reviewed in Libby et al. (2)]. Foam cell apoptosis promotes formation of a necrotic core. Plaque rupture exposes tissue factor to factor VIIa in the circulating blood, which activates the extrinsic coagulation cascade and forms thrombin, which converts fibrinogen to fibrin and clots that can lead to thrombosis and acute coronary syndromes (2).
Inflammation is divided into 2 general stages: initiation and resolution [reviewed in Kumar et al. (3)]. In the initiation phase, arachidonic acid is converted via cyclooxygenases to proinflammatory, prothrombotic, and vasoactive eicosanoids that include prostaglandin (PG) E2, PGF2α, and thromboxane A2 and via 5-lipoxygenase to the proinflammatory leukotriene B4, a potent chemoattractant that recruits leukocytes into tissues [reviewed in Samuelsson (4) and Haeggström and Funk (5)]. As the inflammatory response proceeds, lipid mediator class switching occurs in which PGE2 activates biosynthesis of specialized proresolving lipid mediators (SPMs)–lipoxins, resolvins, protectins and maresins [(6); reviewed in Serhan (7)]. Low-dose aspirin acetylates cyclooxygenase-2, which promotes the biosynthesis of epimeric (also called aspirin-triggered) forms of SPMs, such as 15(R)-lipoxins, 17(R)-resolvins, and 17(R)-protectins (7, 8). Statins also promote 15(R)-epimers of lipoxin A4 (LXA4) via S-nitrosylation of cyclooxygenase-2 (9). SPMs stimulate the resolution of acute inflammation by stopping further neutrophil recruitment to inflamed tissues and by stimulating nonphlogistic infiltration of monocytes that differentiate into macrophages (7). These resolution macrophages then phagocytize and clear apoptotic neutrophils and debris, steps that are key to resolution and prevention of chronic inflammation [(7); reviewed in Tabas and Glass (10)].
Human participants with chronic inflammatory diseases of cystic fibrosis, asthma, and localized aggressive periodontitis have lower levels of SPMs compared with controls without these diseases (11–13). Participants with peripheral arterial disease have lower levels of aspirin-triggered LXA4 (AT-LXA4) compared with healthy volunteers (14). Together, these results suggest that levels of SPMs may be lower in chronic inflammatory diseases in humans. Assessment of SPM in patients with coronary artery disease (CAD) has not been addressed and it is not known whether SPM production is associated with plaque progression or composition. On this basis, we hypothesized that patients with CAD may have low levels of SPMs and, thus, be unable to resolve local acute inflammation that may give rise to chronic uncontrolled inflammation in the arterial wall.
The precursors of SPMs are n-3 fatty acids. E-series resolvins (RvEs) are derived from eicosapentaenoic acid (EPA), and D-series resolvin (RvD), maresin (MaR), and protectin (PD) are derived from docosahexaenoic acid (DHA) by using lipoxygenase pathways (7). n-3 fatty acids have been shown to lower the risk of cardiovascular events. For example, in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto trial, post–myocardial infarction participants who were randomly assigned to 850 mg n-3 fatty acids daily for 3.5 yr had a significant 30% reduction in CVD mortality compared with those participants who were randomly assigned to placebo (15). Recently, Barden et al. (16) reviewed studies that indicated that SPMs are present in peripheral blood and that RvE1 (5S,12R,18R-trihydroxy-eicosa-6Z,8E,10E,14Z,16E-pentaenoic acid), 18-HEPE (18-hydroxy-5Z,8Z,11Z,14Z,216E-eicosapentaenoic acid), 17-HDHA (17-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid), and 14-HDHA (14-hydroxy-4Z,7Z,10Z,12E,16Z,19Z-docosahexaenoic acid) each increase after 5 d of n-3 fatty acid supplementation, with 2.4 g of n-3 fatty acids daily (17). Using whole blood, Dona et al. (18) reported that RvE1 decreases cell adhesion, reduces leukocyte rolling, and blocks platelet aggregation to ADP receptor. These findings suggest mechanisms by which SPMs may lower risk of CVD and, thus, underlie beneficial actions of their precursors, n-3 fatty acids, in reducing CVD risk. Currently, there are no long-term studies on the impact of Lovaza at 4 capsules daily (3.36 g EPA and DHA). This dose is prescribed for lowering triglyceride levels (19, 20). Therefore, we tested in participants with CAD whether long-term EPA and DHA supplementation leads to increases in SPMs. To this end, we profiled SPMs in patients with CAD, examined changes after treatment with Lovaza (an n-3 fatty acid), and assessed the role of these SPMs in promoting clot resolution as measured by macrophage phagocytosis of clot particles.
MATERIALS AND METHODS
Participants
Six male participants with stable CAD were part of the Slowing Heart Disease with Lifestyle and n-3 Fatty Acids (HEARTS) clinical trial in which participants were randomly assigned to either open-label Lovaza 4 capsules daily (3360 mg EPA and DHA daily; n = 3; mean age 62.9 ± 11.8 yr) for 365 d or no Lovaza treatment (n = 3; mean age 56.3 ± 11.9 yr). The trial was not blinded nor was it placebo controlled. All participants were receiving a statin and aspirin. The primary end point of the trial was to examine the effect of n-3 fatty acids on the progression of fatty, fibrous, and calcified plaque in coronary arteries. Results of the primary end point will be reported elsewhere. The protocol was approved by the Beth Israel Deaconess Medical Center Institutional Review Board, and all participants gave written informed consent. Each 1-g capsule of Lovaza contained ∼465 mg EPA and ∼375 mg DHA along with 4 mg α-tocopherol, gelatin, glycerol, and water (https://clinicaltrials.gov#NCT01624727).
Targeted lipid mediator metabololipidomics
Blood was collected in K2-EDTA tubes and centrifuged, and plasma was immediately stored at −80°C. Samples for liquid chromatography–tandem mass spectrometry (LC-MS/MS)–based metabololipidomics from 3 control and 3 Lovaza participants were extracted by using solid-phase (C-18) (21) injected into a LC-MS/MS Qtrap 6500 (AB Sciex, Framingham, MA, USA) that was equipped with a Shimadzu SIL-20AC autoinjector, LC-20AD binary pump (Shimadzu, Kyoto, Japan), and an Agilent Eclipse Plus C18 column (100 × 4.6 mm × 1.8 μm). To identify and quantify lipid mediators (LMs), multiple reaction monitoring was used with signature ion fragments (m/z) for each molecule. Identification was conducted by using published criteria with >6 diagnostic ions and retention time matching of standards (21). Complete stereochemistry of each of the SPMs, that is, RvD1 (7S,8R,17S-trihydroxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid) and RvE1, are determined and given throughout this article and in Colas et al. (21).
Human macrophages
Human peripheral blood mononuclear cells from deidentified healthy human volunteers from the Children’s Hospital Boston blood bank were isolated by density-gradient, Ficoll-Histopaque isolation, and monocytes were purified by using monocyte isolation kit (StemCell Technologies), which yielded a 93–97% CD14 population (22). These monocytes were then cultured for 7 d in RPMI 1640 (10% human serum) and were differentiated to macrophages by using 20 ng/ml granulocyte-macrophage colony-stimulating factor.
Phagocytosis of hemostatic clot
Human whole blood from healthy human volunteers (Protocol 1999P001297; Partners Human Research Committee) was collected 5 times without anticoagulant and allowed to coagulate for 4 h at room temperature. Serum was removed by centrifugation (3000 rpm, 10 min, room temperature), and clots were washed with PBS+/+, then gently dispersed with a small glass pestle and passed through a 70-µm filter. Clot-derived particles—the filtrate that consisted of platelets and cross-linked fibrin particles—were subsequently labeled with wheat germ agglutinin that was conjugated to Alexa Fluor 488 (30 min, room temperature; Thermo Fisher Scientific Life Sciences, Waltham, MA, USA). Unattached dye was removed by centrifugation (3000 rpm, 10 min at room temperature), and supernatants were removed and the pellets washed twice with PBS+/+ and centrifuged (3000 rpm, 10 min). The pellet, which contained labeled clot particles, was then resuspended in PBS+/+. Phagocytosis was carried out as described elsewhere (22). Macrophages were plated in 96-well plates (50,000 cells/well) and incubated with aspirin-triggered resolvin D3 (AT-RvD3) and aspirin-triggered protectin D1 (AT-PD1) at concentrations that ranged from 1 pM to 100 nM for 15 min at 37°C and run in triplicate. Because maximal responses were obtained at 1 nM, macrophages were incubated with RvD1, aspirin-triggered resolvin D1 (AT-RvD1), AT-RvD3, AT-PD1, aspirin-triggered lipoxin B4 (AT-LXB4), PGF2α, and a mix of all aspirin-triggered SPMs (AT-SPMs) at 1 nM in triplicate each for 15 min at 37°C before addition of clot particles. At 60 min, macrophages were washed with PBS to remove nonphagocytosed clot particles, and extracellular fluorescence was quenched by using Trypan blue. Fluorescence was determined by using a SpectraMax M3 plate reader (Molecular Devices, Sunnyvale, CA, USA) monitoring fluorescence emission at 419 nm, and representative images were obtained by using a BZ9000 microscope (Keyence, Itasca, IL, USA).
Statistical analysis
Continuous variables were compared by using paired or unpaired Student’s t tests and categorical variables with χ2 tests. Principal component analysis was performed by using SIMCA 13.0.3 (Umetrics, Umea, Sweden) with mean centering and unit variance scaling. A value of P ≤ 0.05 was considered statistically significant. Statistical calculations were performed by SPSS (22.0) for Windows (IBM, Armonk, NY, USA).
RESULTS
Patients with CAD have bioactive circulating levels of LMs
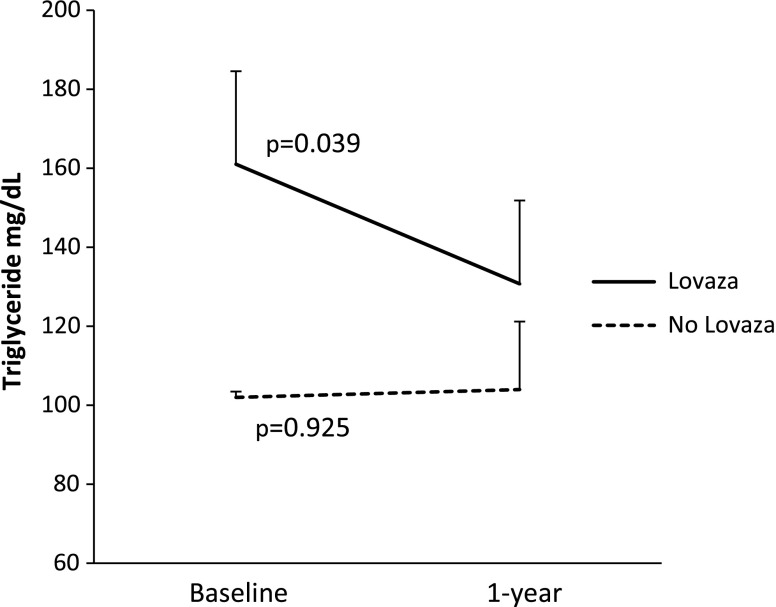
Because Lovaza is a pharmacologic preparation of n-3 fatty acids, EPA and DHA, it was of interest to investigate LMs—this term includes eicosanoids and SPMs—in plasma from patients with CAD to assess the potential impact of LMs in human participants. To this end, we carried out targeted LM metabololipidomics by using a LC-MS/MS approach. Table 1 shows the clinical characteristics of the participants at 1 yr. There were no statistically significant differences between the 2 groups, a finding that suggests that random assignment was successful. Figure 1 shows that those receiving Lovaza had a significant reduction in triglyceride level at 1 yr (P = 0.039) compared with those who did not receive Lovaza, who had no change (P = 0.925). This finding is expected on the basis of the current U.S. Food and Drug Administration–approved indication for Lovaza, which is to lower triglyceride levels (19, 20).
TABLE 1.
Clinical characteristics of study participants at 1 yr
| Variable | CAD | CAD + Lovaza | P |
|---|---|---|---|
| Demographics | |||
| Age (yr, mean ± sd) | 56.3 ± 11.9 | 62.9 ± 11.8 | 0.54 |
| Male [N (%)] | 3 (100.0) | 3 (100.0) | 1.0 |
| White [N (%)] | 3 (100.0) | 3 (100.0) | 1.0 |
| Anthropometrics and vital signs (mean ± sd) | |||
| Weight (kg) | 104.3 ± 22.1 | 86.8 ± 4.7 | 0.25 |
| Body mass index (kg/m2) | 30.8 ± 5.6 | 28.2 ± 1.2 | 0.46 |
| Systolic blood pressure (mmHg) | 120.4 ± 7.0 | 113.0 ± 8.5 | 0.31 |
| Diastolic blood pressure (mmHg) | 73.6 ± 4.3 | 70.0 ± 7.3 | 0.50 |
| Cardiac risk factors [n/N (%)] | |||
| Hypertension | 2/3 (66.7) | 3/3 (100.0) | 0.42 |
| Diabetes | 0/0 (0.0) | 0/0 (0.0) | |
| High LDL-C | 1/3 (33.3) | 2/3 (66.7) | 0.52 |
| Low HDL-C | 2/3 (66.7) | 3/3 (100.0) | 0.42 |
| Hypertriglyceridemia | 3/3 (100.0) | 2/3 (66.7) | 0.42 |
| Smoking | 2/3 (66.7) | 0/0 (0.0) | 0.18 |
| Family history of CVD | 2/3 (66.7) | 2/3 (66.7) | 1.0 |
| Peripheral arterial disease | 0/0 (0.0) | 0/0 (0.0) | |
| Inclusion criteria [n/N (%)]a | |||
| Myocardial infarction | 1/3 (33.3) | 2/3 (66.7) | 0.52 |
| Stable angina | 1/3 (33.3) | 1/3 (33.3) | 1.0 |
| Abnormal stress test | 2/3 (66.7) | 2/3 (66.7) | 1.0 |
| Coronary angioplasty or stent | 3/3 (100.0) | 1/3 (33.3) | 0.18 |
| Laboratory values (mean ± sd) | |||
| Total cholesterol (mg/dl) | 135.0 ± 41.8 | 151.3 ± 16.2 | 0.56 |
| LDL-C (mg/dl) | 63.0 ± 26.9 | 85.7 ± 3.2 | 0.22 |
| HDL-C (mg/dl) | 51.3 ± 18.9 | 40.3 ± 7.2 | 0.40 |
| Triglycerides (mg/dl) | 103.3 ± 30.0 | 126.7 ± 37.1 | 0.45 |
| Glucose (mg/dl) | 93.0 ± 5.6 | 95.0 ± 3.6 | 0.63 |
| HbA1c (%) | 5.6 ± 0.3 | 5.6 ± 0.1 | 0.68 |
| eGFR (ml/min/1.73 m2) | 88.3 ± 6.5 | 82.7 ± 22.8 | 0.70 |
| Creatinine clearance (ml/min) | 130.2 ± 42.6 | 97.5 ± 35.6 | 0.37 |
| Ethanol (U/wk) | 1.4 ± 1.4 | 9.0 ± 6.3 | 0.11 |
| Medications, n/N (%) | |||
| Aspirin 81 mg | 1/3 (33.3) | 1/3 (33.3) | 1.0 |
| Aspirin 162 mg | 0/0 (0.0) | 1/3 (33.3) | 0.42 |
| Aspirin 325 mg | 2/3 (66.7) | 1/3 (33.3) | 0.52 |
| Statins | |||
| Simvastatin | 1/3 (33.3) | 2/3 (66.7) | 0.52 |
| Atorvastatin | 1/3 (33.3) | 1/3 (33.3) | 1.0 |
| Rosuvastatin | 1/3 (33.3) | 0/0 (0.0) | 0.42 |
| Diuretics | 0/0 (0.0) | 1/3 (33.3) | 0.42 |
| β-Blocker | 2/3 (66.7) | 2/3 (66.7) | 1.0 |
| Angiotensin-converting enzyme inhibitor | 2/3 (66.7) | 2/3 (66.7) | 1.0 |
| Nitroglycerine | 2/3 (66.7) | 1/3 (33.3) | 0.52 |
| Plavix | 1/3 (33.3) | 1/3 (33.3) | 1.0 |
| Warfarin | 0/0 (0.0) | 1/3 (33.3) | 0.42 |
| Isosorbide mononitrate | 1/3 (33.3) | 0/0 (0.0) | 0.42 |
| Dose of statin and aspirin (mean ± sd) | |||
| Aspirin (mg) | 243.7 ± 140.9 | 189.3 ± 124.3 | 0.64 |
| Atorvastatin or equivalent (mg) | 46.7 ± 30.6 | 43.3 ± 35.1 | 0.91 |
Clinical characteristics of patients with CAD who received aspirin or aspirin and Lovaza (3360 mg EPA and DHA daily) at 1-yr follow-up; n = 3. eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c. a May have >1.
Figure 1.
Levels of triglyceride at 1 yr compared with baseline in participants who received Lovaza compared with those who did not receive Lovaza. Values are expressed as means ± sem.
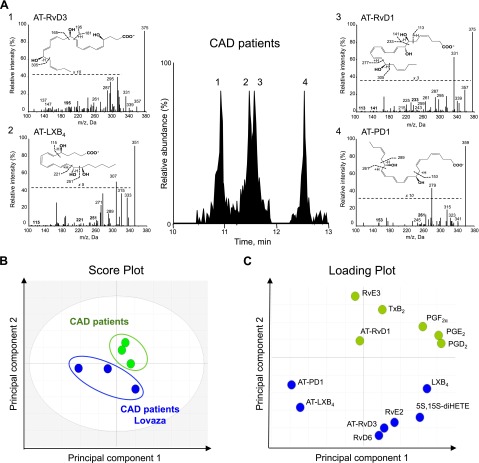
Eicosanoids and SPMs from DHA, EPA, and arachidonic acid functional metabolomes were identified (Table 2). Figure 2A shows representative multiple reaction monitoring chromatograms of selected ion pairs for AT-RvD1, AT-RvD3, AT-LXB4, and AT-PD1 along with representative MS/MS spectra and diagnostic ions employed for their identification. Both groups of participants had the presence of AT-RvD1, AT-PD1, RvD6 (4S,17S-dihydroxy-5E,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid), RvE2 (5S,18R-dihydroxy-eicosa-6E,8Z,11Z,14Z,16E-pentaenoic acid), RvE3 (17R,18R-dihydroxy-eicosa-5Z,8Z,11Z,13E,15E-pentaenoic acid), and LXB4 as well as eicosanoid cyclooxygenase products PGD2, PGE2, PGF2α and thromboxane B2 (Table 2). In addition, both groups had absence of mediators shown to be present in healthy participants (17, 21, 23), namely specific SPMs that included RvD1, RvD2 (7S,16R,17S-trihydroxydocosa-4Z,8E,10Z,12E,14E,19Z-hexaenoic acid), RvD3 (4S,11R,17S-trihydroxydocosa-5Z,7E,9E,13Z,15E,19Z-hexaenoic acid), RvD5 (7S,17S-dihydroxydocosa-4Z,8E,10Z,13Z,15E,19Z-hexaenoic acid), and RvE1. Patients with CAD who received Lovaza had levels of the proresolving and anti-inflammatory mediators, RvD6 and AT-PD1, that were twice as high as well as RvE2 that was ∼5 fold higher, and lower levels of prostaglandins compared with patients with CAD who did not receive Lovaza for 1 yr. Of interest, the proresolving and anti-inflammatory mediators, AT-RvD3 and AT-LXB4, were absent in patients with CAD who did not receive Lovaza but were present in those who did receive Lovaza. Amounts of AT-RvD1 were apparently unaffected by treatment with Lovaza in these participants (Table 2). Note that SPMs identified herein were in their bioactive concentration range as determined with isolated human cells and with animal disease models in vivo [reviewed in Serhan (7)].
TABLE 2.
Human plasma LMs from patients with CAD
| LM | Q1 | Q3 | CAD | CAD + Lovaza |
|---|---|---|---|---|
| DHA bioactive metabolome | ||||
| RvD1 | 375 | 215 | — | — |
| RvD2 | 375 | 215 | — | — |
| RvD3 | 375 | 181 | — | — |
| RvD5 | 359 | 199 | — | — |
| RvD6 | 359 | 159 | 11.0 ± 3.4 | 22.5 ± 4.6 |
| AT-RvD1 | 375 | 141 | 14.6 ± 2.7 | 12.9 ± 2.6 |
| AT-RvD3 | 375 | 181 | — | 7.1 ± 3.7 |
| PD1 | 359 | 153 | — | — |
| AT-PD1 | 359 | 153 | 3.0 ± 0.5 | 6.0 ± 1.9 |
| MaR1 | 359 | 250 | — | — |
| EPA bioactive metabolome | ||||
| RvE1 | 349 | 161 | — | — |
| RvE2 | 333 | 115 | 8.2 ± 8.2 | 44.8 ± 38.6 |
| RvE3 | 333 | 201 | 69.0 ± 11.6 | 61.7 ± 2.8 |
| Arachidonic acid bioactive metabolome | ||||
| LXA4 | 351 | 115 | — | — |
| LXB4 | 351 | 115 | 453.9 ± 88.4 | 369.3 ± 105.2 |
| 5S,15S-diHETE | 335 | 235 | 45.0 ± 4.1 | 48.2 ± 10.5 |
| AT-LXA4 | 351 | 115 | — | — |
| AT-LXB4 | 351 | 221 | — | 20.5 ± 4.3 |
| LTB4 | 335 | 195 | — | — |
| PGD2 | 351 | 189 | 1.7 ± 0.2 | 0.9 ± 0.5 |
| PGE2 | 351 | 189 | 3.1 ± 0.3 | 1.7 ± 0.9 |
| PGF2α | 353 | 193 | 3.1 ± 0.7 | 1.0 ± 0.5 |
| TxB2 | 369 | 169 | 3.2 ± 1.6 | 2.1 ± 1.4 |
Results are expressed as picograms per milliliter; means ± sem; n = 3. Limit of detection, ∼0.1 pg. Human plasma from patients with CAD who received aspirin or aspirin and Lovaza (3360 mg EPA and DHA daily for 1 yr) were extracted, and LM levels were investigated by LM metabololipidomics (see Materials and Methods for details). 5S,15S-diHETE, 5S,15S‐dihydroxy‐eicosatetraenoic acid; LTB4, leukotriene B4; TxB2, thromboxane B2; Q1, M-H (parent ion); Q3, diagnostic ion in the MS-MS (daughter ion). LMs with no CAD value are below the limit of detection. Those SPMs absent in patients with CAD who did not receive Lovaza were present in healthy volunteers from a prior study that used analysis conditions identical to those of the current report, with the exception of PD1, MaR1, LXA4, and AT-LXA4, which were absent in both healthy volunteers and patients with CAD (21).
Figure 2.
Human plasma bioactive LM profiles from patients with CAD taking aspirin are modified by Lovaza intake. Human plasma from patients with CAD taking aspirin or aspirin and Lovaza (3360 mg EPA and DHA daily for 1 yr) were extracted and LM levels were investigated by using LM metabololipidomics (see Materials and Methods for details). A) Representative multiple reaction monitoring chromatograms of selected ion pairs for AT-RvD1, AT-RvD3, AT-LXB4, and AT-PD1 are reported along with representative MS/MS spectra and diagnostic ions employed for their identification; representative of n = 6. B, C) Principal component analysis 2-dimensional score plot (B) and its corresponding loading plot (C). Gray ellipse in the score plot (B) denotes 95% CI regions; n = 6.
Lovaza shifts LM-SPM profiles
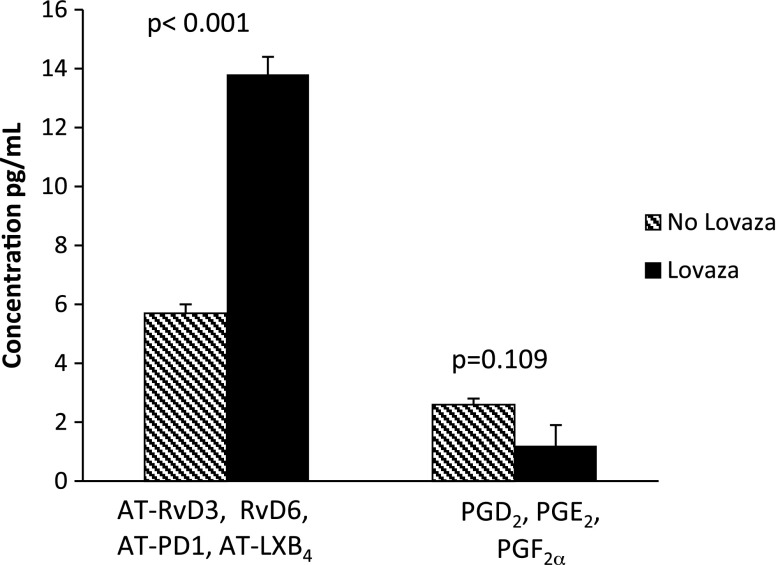
Having identified and established the profile of plasma LMs-SPMs in patients with CAD, we next investigated whether Lovaza intake was causally linked to changes in LM-SPM profiles. To this end, we conducted unbiased principle component analysis (Fig. 2B, C), with results obtained from LC-MS/MS profiling. The 2 principal components of the score plot (Fig. 2B) showed a clear separation between patients with CAD who did not receive Lovaza compared with patients with CAD who did receive Lovaza. The corresponding loading plot (Fig. 2C) revealed a positive relationship for patients with CAD who received Lovaza with AT-RvD3, RvD6, AT-PD1, and AT-LXB4. When these 4 SPMs were combined, those who received Lovaza had significantly higher levels compared with those who did not receive Lovaza (P < 0.001; as indicated in Fig. 3). Those receiving Lovaza also had lower levels of prostaglandins compared with those not receiving Lovaza, a finding that approached statistical significance (P = 0.109; Fig. 3).
Figure 3.
Levels of combined AT-RvD3, RvD6, AT-PD1, and AT-LXB4, and combined prostaglandins at 1 yr in participants who received Lovaza compared with those who did not receive Lovaza. Values are expressed as means ± sem.
Lovaza-stimulated SPMs enhance human macrophage clot phagocytosis
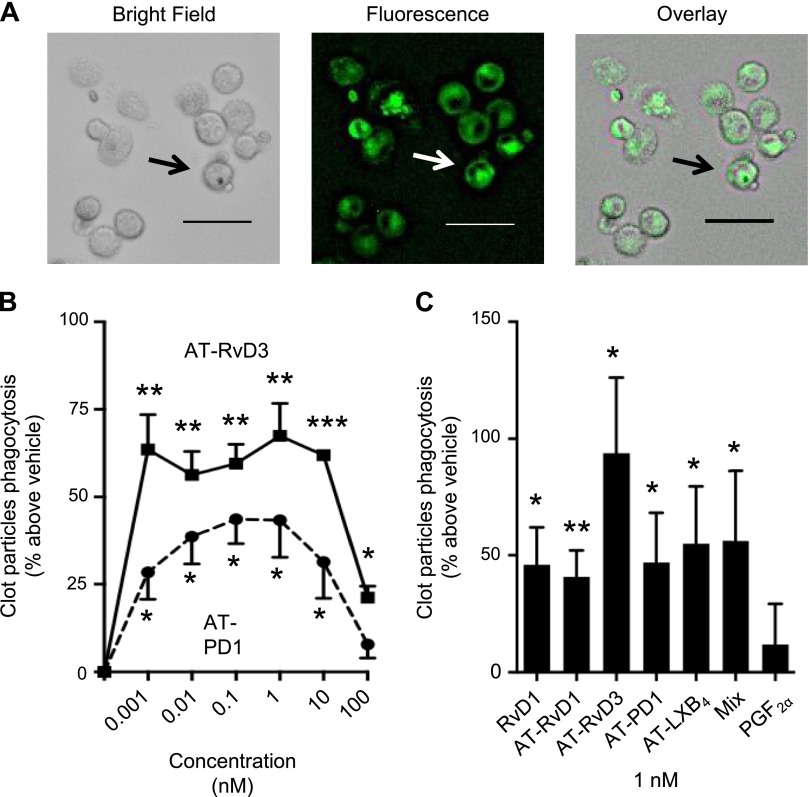
As acute coronary syndromes are caused by plaque rupture and subsequent thrombotic events (2), we tested whether the specific SPMs identified in patients with CAD who received Lovaza affect blood clot uptake within macrophages that are exposed to fluorescent-labeled clot components. Macrophages phagocytized labeled clots (Fig. 4A). Because AT-PD1 and AT-RvD3 were increased in patients with CAD who received Lovaza, we tested 1 pM to 100 nM of these 2 SPMs (Fig. 4B). In the presence of these SPMs, phagocytosis was increased (∼27–70%) in a bell-shaped dose response that likely reflects engagement of different receptors. Because AT-PD1 and AT-RvD3 enhanced clot uptake with a maximum increase in phagocytosis obtained at 1 nM of these SPMs (Fig. 4B), we compared the activity of these SPMs with that of RvD1, AT-RvD1, AT-RvD3, AT-PD1, AT-LXB4, or a mix of all 4 AT-SPMs at the same concentration (Fig. 4C). Each SPM significantly increased macrophage phagocytosis of clots by an average of ∼50%. For direct comparison, the proinflammatory mediator, PGF2α, did not stimulate clot uptake.
Figure 4.
Bioactive SPMs identified in patients with CAD who received Lovaza display potent bioactions in clot remodeling in vitro. A) Human macrophages (1 × 105 cells/well) were incubated with fluorescently labeled clot particles (60 min, 37°C, pH 7.45), and fluorescence was assessed by using a BZ9000 microscope equipped with a ×20 objective. Representative of n = 5 separate macrophage preparation and 4 fields/well. B) Representative dose response for macrophages (5 × 104 cells/well) incubated with AT-RvD3 and AT-PD1 at indicated doses and fluorescently labeled clot particles. Results are means ± sem, d 3. C) Human macrophages (5 × 104 cells/well) were incubated with 1 nM RvD1, AT-RvD1, AT-RvD3, AT-PD1, AT-LXB4, a mix of AT-SPMs, or PGF2α and fluorescently labeled clot particles. Fluorescence was assessed by using a SpectraMax M3 plate reader. Results are means ± sem, n = 4–8 separate macrophage preparation. *P < 0.05; **P < 0.01; ***P < 0.001 vs. vehicle. Scale bars, 50 μm.
DISCUSSION
In the present study, we identified LM-SPM profiles by using LM metabololipidomics in patients with CAD. In patients with CAD who did not receive Lovaza, RvD1, RvD2, RvD3, RvD5, RvE1, AT-RvD3, and AT-LXB4 were absent; these mediators are present in healthy participants (17, 21, 23). Lack of these specific SPMs in patients with CAD suggests a potential decrease in resolution capacity for acute inflammation or failed local resolution that could presumably lead to chronic or uncontrolled inflammation in these patients. Compared with patients with CAD who did not receive Lovaza, patients with CAD who did receive Lovaza 3.36 g for 12 mo had the presence of AT-LXB4 and AT-RvD3 as well as higher levels of AT-PD1, RvD6, and RvE2, which suggests potential improvements in resolution LM and specific SPM biosynthesis. Of note, AT-RvD1 did not change in these individuals, a finding that suggests that Lovaza stimulates or enhances certain selective SPM pathways. Healthy volunteers (i.e., without CAD) before and after low-dose n-3 fatty acids and aspirin had lipoxins and resolvins present before supplementation; their levels increased after n-3 fatty acid supplementation (21). By using LC-MS/MS, Sasaki et al. (24) identified SPMs in urine of healthy volunteers who were not taking n-3 fatty acid supplements, and Mas et al. (25) identified SPMs in plasma of healthy volunteers after n-3 fatty acid supplementation for 3 wk. These results suggest that healthy volunteers produce circulating proresolution mediators, whereas patients with CAD examined in the present study showed an absence of ≥1 proresolution mediators that were partially restored by treatment with n-3 fatty acids (Lovaza), the precursors of SPMs.
These results are noteworthy given the known bioactions of SPMs, which include proresolving and anti-inflammatory actions, without causing immunosuppression [reviewed in Serhan (7)], and that several SPMs—AT-RvD3, AT-PD1, RvE2, and AT-LXB4—were increased by Lovaza (Table 2; Figs. 2 and 3). These trends in SPMs seem to be in line with a reduction in proinflammatory state for Lovaza in patients with CAD as evidenced by reductions in prostaglandins, which are classic proinflammatory mediators, in the Lovaza-treated participants in the current study. Because SPMs reduce polymorphonuclear leukocyte infiltration in animal disease models (7) and individual human cells (26), the mechanism for reduction in prostaglandins with Lovaza in humans may result from the actions of SPMs. Although SPMs have a short half-life at sites of inflammation and are inactivated by leukocytes (27), daily Lovaza reaches steady-state within 1 mo (28); therefore, a continuous supply of substrate could account for the increase in levels of plasma SPMs observed herein and in prior studies (21). Compared with Colas et al. (21), who used LC-MS/MS methods similar to the current study and reported the short-term response of SPM profiles at 4 h in healthy volunteers who received 1 low dose of n-3 fatty acids (1 g) followed 2 h later by aspirin 81 mg, our patients with CAD who received long-term, high-dose (3.36 g) n-3 fatty acid supplementation and aspirin for 1 yr had higher levels of RvD6 (0.2 ± 0.1 vs. 22.5 ± 4.6 pg/ml, respectively), AT-RvD1 (0.1 ± 0.1 vs. 12.9 ± 2.6 pg/ml, respectively), AT-RvD3 (0.1 ± 0.1 vs. 7.1 ± 3.7 pg/ml, respectively), AT-PD1 (1.2 ± 0.5 vs. 6.0 ± 1.9 pg/ml, respectively), RvE2 (4.9 ± 2.4 vs. 44.8 ± 38.6 pg/ml, respectively), RvE3 (11.8 ± 3.7 vs. 61.7 ± 2.8 pg/ml, respectively), LXB4 (0.2 ± 0.1 vs. 369.3 ± 105.2 pg/ml, respectively), and AT-LXB4 (0.3 ± 0.2 vs. 20.5 ± 4.3 pg/ml, respectively). These findings suggest that 1-yr supplementation with high-dose n-3 fatty acids leads to higher levels of SPMs compared with short-term, low-dose supplementation. Of note, our participants were also receiving long-term treatment with aspirin and statin drugs, both of which have been shown to stimulate the biosynthesis of AT-SPMs (7, 8) and could also account for higher levels of SPMs observed in our participants compared with those of Colas et al. (21). In whole blood, RvE1 has been shown to be active over a 4-h time period, during which it decreases cell adhesion, reduces leukocyte rolling, and blocks platelet aggregation to ADP receptor (18), all of which may decrease chronic inflammation in atherosclerosis and, thus, underlie potential beneficial actions of their precursors, n-3 fatty acids, in reducing CVD risk. Amounts of SPMs obtained in samples in the current study were commensurate with their picogram and nanogram actions with isolated human leukocytes and animal disease models [reviewed in Serhan (7)]. SPMs may thus reduce pathogenic mechanisms that can contribute to progression of local atherosclerotic lesions at specific points within pathogenesis; hence, the finding that restoration of specific SPMs in patients with CAD to levels within their bioactive concentration range (Fig. 4) may provide novel therapeutic approaches in cardiovascular medicine.
The suggestion that SPMs could resolve inflammation in the context of atherosclerosis is supported by results from animal models and isolated human cells (29–31). For example, with human vascular smooth muscle cells, RvD1 and RvD2 inhibit vascular smooth muscle cell proliferation and migration, monocyte adhesion, superoxide production, and proinflammatory gene expression in a dose-dependent manner, with an apparent inhibitory concentration for 50% reduction of ∼0.1–1 nM (29). In addition, MaR1 decreased TNF-α–induced monocyte adhesion and production of reactive oxygen species via up-regulation of cAMP and down-regulation of NF-κB in cultured human saphenous vein endothelial cells and vascular smooth muscle cells in a time-dependent manner (30). Macrophage-specific overexpression of human 15-lipoxygenase led to biosynthesis of LXA4, RvD1, and PD1 and reduced plaque formation in apoE/lipoxygenase−/−-deficient mice (31). An inverse correlation between plasma AT-LXA4 and peripheral atherosclerosis was found in a cross-sectional human study (14). Results from 2 human case-control studies have provided support for a protective role of variants in the gene for 15-lipoxygenase type 1 against CAD in humans (32, 33). In balloon injury of the femoral artery in rabbits, RvD1, RvD5, LXB4, and MaR1 were produced during vascular injury (29). RvD2 reduced cell proliferation by ∼50% and leukocyte recruitment by ∼40% to injured arteries and neointimal hyperplasia by 29% at 28 d after injury (29). In a carotid artery ligation model in mice, RvD2 and MaR1 reduced neutrophil and macrophage recruitment, increased polarization of M2 (anti-inflammatory) macrophages in the arterial wall, and attenuated intimal hyperplasia (34). Liposomal-delivered RvD1 improved fractional shortening post–myocardial infarction by decreasing fibrosis, thereby delaying the onset of heart failure in mice (35). In rabbits, topical RvE1 application to the periodontium with periodontal disease decreased aortic plaque size and formation (36). Together, these findings suggest that both resolvin D- and E-series may benefit injured vessels to reduce atherosclerosis and improve remodeling post–myocardial infarction.
Another finding in the current study is that the specific SPMs that were increased by Lovaza in patients with CAD, namely AT-RvD3 and AT-PD1, significantly stimulated human macrophages to phagocytose human blood clots, whereas the bioactive proinflammatory mediator, PGF2α, did not stimulate the uptake of blood clots by human macrophages. This finding suggests that SPMs may play an intraluminal role in clot resolution by helping to remove thrombus. Along these lines, Dona et al. (18) showed that RvE1 inhibits ADP and thromboxane receptor agonist-induced human platelet aggregation in whole blood, and Kain et al. (35) reported that RvD1 reduces myocardial fibrosis after myocardial infarction in mice. Thus, the present results warrant further consideration of the role of proresolution mediators in clot remodeling.
Our finding that Lovaza increases certain SPMs may provide an explanation for some of the beneficial effects of n-3 fatty acids, the precursors of SPMs (7), in alleviating vascular inflammation. For example, high plasma levels of n-3 fatty acids have been associated with slower progression of coronary artery atherosclerosis as measured by intravascular ultrasound (37). A meta-analysis of 11 randomized, double-blind, placebo-controlled trials of 15,348 patients with a history of cardiovascular disease who were taking at least 1 g/d of an n-3 fatty acid supplement for ≥1 yr showed a statistically significant protective effect of n-3 fatty acid supplement on cardiac death [relative risk (RR), 0.68; 95% confidence interval (CI), 0.56–0.83], sudden death (RR, 0.67; 95% CI, 0.52–0.87), and myocardial infarction (RR, 0.75; 95% CI, 0.63–0.88) (38). As mentioned earlier, in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto trial, those post–myocardial infarction participants who were randomly assigned to Lovaza 840 mg daily for 3.5 yr had a significant 30% reduction in CVD mortality compared with those who received placebo (15). In the Cardiovascular Health Study, a prospective cohort study of 2692 U.S. adults free of CAD at baseline, higher plasma levels of n-3 fatty acids were associated with a 27% reduction in total mortality [hazard ratio (HR), 0.73; 95% CI, 0.61–0.86; P trend ≤ 0.008], a finding attributable to fewer cardiovascular deaths (39). In the observational Multi-Ethnic Study of Atherosclerosis, higher plasma EPA and DHA levels were associated with fewer cardiovascular events (40). These beneficial findings, and more recent studies reviewed in Barden et al. (16), emphasize the importance of further human studies of SPMs and n-3 fatty acids.
It should be noted that not all clinical trials with n-3 fatty acids demonstrated a benefit. In the Outcome Reduction with Initial Glargine Intervention (ORIGIN), a double-blind, placebo-controlled study in which 12,536 patients at high risk for cardiovascular events and with impaired fasting glucose and impaired glucose tolerance or diabetes were randomly assigned in a 2×2 factorial design to supplementation with n-3 fatty acids (465 mg EPA and 375 mg DHA Lovaza, formerly called Omacor) or placebo (1 g of olive oil), as well as to receive either insulin glargine or standard care, the primary outcome was not significantly decreased among patients receiving n-3 fatty acids compared with those receiving placebo; cardiovascular mortality (HR, 0.98; 95% CI, 0.87–1.10; P = 0.72), major vascular events (HR, 1.01; 95% CI, 0.93–1.10; P = 0.81), death from any cause (HR, 0.98; 95% CI, 0.89–1.07; P = 0.63), or death from arrhythmia (HR, 1.10; 95% CI, 0.93–1.30; P = 0.26) over a median follow-up of 6.2 yr (41). Lack of benefit in ORIGIN may have been a result of the beneficial actions of the 1 g placebo olive oil capsules, a dose higher than the 840 mg EPA and DHA capsule. A meta-analysis of trials comparing olive oil with comparable doses of n-3 fatty acids showed that daily consumption of olive oil interventions at doses ranging from 1 to 50 mg—doses that are lower than those used in ORIGIN—resulted in a significantly more pronounced decrease in C-reactive protein (P < 0.0001; n = 15 trials) and IL-6 (P < 0.04; n = 7 trials) compared with controls, which included n-3 fatty acids, respectively (42). Flow-mediated brachial dilatation as a measure of endothelial function was also significantly more increased in those who received olive oil compared with controls (P < 0.002; n = 8 trials) (42). Additional potential mechanisms for the beneficial effects of olive oil include a suppression of reactive oxygen species–mediated, NF-κB–dependent matrix metalloproteinase-9 and cyclooxygenase-2 expression (43) and an increase in HDL cholesterol (44) from the polyphenols of olive oil. Therefore, olive oil may improve cardiovascular outcomes and mortality by decreasing inflammation and improving endothelial function, and, thus, the dose in ORIGIN may not be a useful placebo.
n-3 fatty acids have also been shown to be beneficial in other chronic inflammatory diseases, such as rheumatoid arthritis and inflammatory bowel disease (45). At least 20 randomized, placebo-controlled, double-blind studies of n-3 fatty acids in patients with rheumatoid arthritis have shown benefits that include reduced duration of morning stiffness, reduced number of tender or swollen joints, reduced joint pain, reduced time to fatigue, increased grip strength, and decreased use of nonsteroidal anti-inflammatory drugs [reviewed in Calder (45), Yacoubian and Serhan (46), Cleland et al. (47), Lee et al. (48), and Leeb et al. (49)]. On this basis, n-3 fatty acids are recommended for rheumatoid arthritis (50).
In conclusion, the present findings identified specific SPMs in human participants and suggest that patients with CAD have lower levels and/or absence of certain SPMs, whereas treatment with Lovaza seemed to restore formation of several SPMs. The restored SPMs, AT-RvD3, AT-LXB4, RvD6, AT-PD1, and RvE2, promote macrophage phagocytosis of blood clots in vitro. Low vascular SPMs could lead to chronic vascular inflammation and predispose to coronary atherosclerosis and thrombosis. Current clinical U.S. Food and Drug Administration–approved indication for Lovaza is to lower levels of triglyceride [(19); reviewed in De Caterina (20)]. Our results suggest that Lovaza may also have benefits beyond triglyceride lowering. Further human studies are warranted, and our results now implicate examining the impact of Lovaza on SPM levels and their role in the extent and severity of vascular disease and local tissue inflammation.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grants P01GM095467 and RO1GM03865 (to C.N.S.), and NIH National Heart, Lung, and Blood Institute Grant P50HL083813 (to F.K.W.). C.N.S. is an inventor of patents (resolvins) assigned to the Brigham and Women’s Hospital, and licensed to Resolvyx Pharmaceuticals. C.N.S. is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. The interests of C.N.S. were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflicts of interest policies. The remaining authors declare no conflicts of interests.
Glossary
- AT-LXB4
aspirin-triggered lipoxin B4
- AT-PD1
aspirin-triggered protectin D1
- AT-RvD1
aspirin-triggered resolvin D1
- AT-RvD3
aspirin-triggered resolvin D3
- AT-SPM
aspirin-triggered specialized proresolving lipid mediator
- CAD
coronary artery disease
- CI
confidence interval
- CVD
cardiovascular disease
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- HR
hazard ratio
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- LM
lipid mediator
- LXA4
lipoxin A4
- LXB4
lipoxin B4
- MaR
maresin
- PG
prostaglandin
- RR
relative risk
- RvD
D-series resolvin
- RvE
E-series resolvin
- SPM
specialized proresolving lipid mediator
REFERENCES
- 1.World Health Organization (rev. January 2015) Cardiovascular diseases (CVDs). Accessed December 11, 2015, at: http://www.who.int/mediacentre/factsheets/fs317/en/
- 2.Libby P., Tabas I., Fredman G., Fisher E. A. (2014) Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 114, 1867–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar V., Abbas A. K., Aster J. C. (2015) Inflammation and repair. In Robbins and Cotran Pathologic Basis of Disease, 9th ed., pp. 69–111, Elsevier/Saunders, Philadelphia [Google Scholar]
- 4.Samuelsson B. (2012) Role of basic science in the development of new medicines: examples from the eicosanoid field. J. Biol. Chem. 287, 10070–10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haeggström J. Z., Funk C. D. (2011) Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 111, 5866–5898 [DOI] [PubMed] [Google Scholar]
- 6.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 [DOI] [PubMed] [Google Scholar]
- 7.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang N., Bermudez E. A., Ridker P. M., Hurwitz S., Serhan C. N. (2004) Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl. Acad. Sci. USA 101, 15178–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnbaum Y., Ye Y., Lin Y., Freeberg S. Y., Nishi S. P., Martinez J. D., Huang M. H., Uretsky B. F., Perez-Polo J. R. (2006) Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation 114, 929–935 [DOI] [PubMed] [Google Scholar]
- 10.Tabas I., Glass C. K. (2013) Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339, 166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karp C. L., Flick L. M., Park K. W., Softic S., Greer T. M., Keledjian R., Yang R., Uddin J., Guggino W. B., Atabani S. F., Belkaid Y., Xu Y., Whitsett J. A., Accurso F. J., Wills-Karp M., Petasis N. A. (2004) Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 5, 388–392 [DOI] [PubMed] [Google Scholar]
- 12.Levy B. D., Bonnans C., Silverman E. S., Palmer L. J., Marigowda G., Israel E.; Severe Asthma Research Program, National Heart, Lung, and Blood Institute (2005) Diminished lipoxin biosynthesis in severe asthma. Am. J. Respir. Crit. Care Med. 172, 824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredman G., Oh S. F., Ayilavarapu S., Hasturk H., Serhan C. N., Van Dyke T. E. (2011) Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1. PLoS One 6, e24422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho K. J., Spite M., Owens C. D., Lancero H., Kroemer A. H., Pande R., Creager M. A., Serhan C. N., Conte M. S. (2010) Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am. J. Pathol. 177, 2116–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GISSI-Prevenzione Investigators (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 354, 447–455 [PubMed] [Google Scholar]
- 16.Barden A. E., Mas E., Mori T. A. (2016) n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr. Opin. Lipidol. 27, 26–32 [DOI] [PubMed] [Google Scholar]
- 17.Barden A., Mas E., Croft K. D., Phillips M., Mori T. A. (2014) Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 55, 2401–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dona M., Fredman G., Schwab J. M., Chiang N., Arita M., Goodarzi A., Cheng G., von Andrian U. H., Serhan C. N. (2008) Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 112, 848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris W. S., Ginsberg H. N., Arunakul N., Shachter N. S., Windsor S. L., Adams M., Berglund L., Osmundsen K. (1997) Safety and efficacy of Omacor in severe hypertriglyceridemia. J. Cardiovasc. Risk 4, 385–391 [PubMed] [Google Scholar]
- 20.De Caterina R. (2011) n-3 fatty acids in cardiovascular disease. N. Engl. J. Med. 364, 2439–2450 [DOI] [PubMed] [Google Scholar]
- 21.Colas R. A., Shinohara M., Dalli J., Chiang N., Serhan C. N. (2014) Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 307, C39–C54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalli J., Serhan C. N. (2012) Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Psychogios N., Hau D. D., Peng J., Guo A. C., Mandal R., Bouatra S., Sinelnikov I., Krishnamurthy R., Eisner R., Gautam B., Young N., Xia J., Knox C., Dong E., Huang P., Hollander Z., Pedersen T. L., Smith S. R., Bamforth F., Greiner R., McManus B., Newman J. W., Goodfriend T., Wishart D. S. (2011) The human serum metabolome. PLoS One 6, e16957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki A., Fukuda H., Shiida N., Tanaka N., Furugen A., Ogura J., Shuto S., Mano N., Yamaguchi H. (2015) Determination of ω-6 and ω-3 PUFA metabolites in human urine samples using UPLC/MS/MS. Anal. Bioanal. Chem. 407, 1625–1639 [DOI] [PubMed] [Google Scholar]
- 25.Mas E., Croft K. D., Zahra P., Barden A., Mori T. A. (2012) Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 58, 1476–1484 [DOI] [PubMed] [Google Scholar]
- 26.Kasuga K., Yang R., Porter T. F., Agrawal N., Petasis N. A., Irimia D., Toner M., Serhan C. N. (2008) Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol. 181, 8677–8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong S., Porter T. F., Lu Y., Oh S. F., Pillai P. S., Serhan C. N. (2008) Resolvin E1 metabolome in local inactivation during inflammation-resolution. J. Immunol. 180, 3512–3519 [DOI] [PubMed] [Google Scholar]
- 28.Arterburn L. M., Hall E. B., Oken H. (2006) Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 83(6, Suppl)1467S–1476S [DOI] [PubMed] [Google Scholar]
- 29.Miyahara T., Runge S., Chatterjee A., Chen M., Mottola G., Fitzgerald J. M., Serhan C. N., Conte M. S. (2013) D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 27, 2220–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee A., Sharma A., Chen M., Toy R., Mottola G., Conte M. S. (2014) The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS One 9, e113480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merched A. J., Ko K., Gotlinger K. H., Serhan C. N., Chan L. (2008) Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22, 3595–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittwer J., Bayer M., Mosandl A., Muntwyler J., Hersberger M. (2007) The c.-292C>T promoter polymorphism increases reticulocyte-type 15-lipoxygenase-1 activity and could be atheroprotective. Clin. Chem. Lab. Med. 45, 487–492 [DOI] [PubMed] [Google Scholar]
- 33.Assimes T. L., Knowles J. W., Priest J. R., Basu A., Borchert A., Volcik K. A., Grove M. L., Tabor H. K., Southwick A., Tabibiazar R., Sidney S., Boerwinkle E., Go A. S., Iribarren C., Hlatky M. A., Fortmann S. P., Myers R. M., Kuhn H., Risch N., Quertermous T. (2008) A near null variant of 12/15-LOX encoded by a novel SNP in ALOX15 and the risk of coronary artery disease. Atherosclerosis 198, 136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akagi D., Chen M., Toy R., Chatterjee A., Conte M. S. (2015) Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 29, 2504–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kain V., Ingle K. A., Colas R. A., Dalli J., Prabhu S. D., Serhan C. N., Joshi M., Halade G. V. (2015) Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 84, 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasturk H., Abdallah R., Kantarci A., Nguyen D., Giordano N., Hamilton J., Van Dyke T. E. (2015) Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscler. Thromb. Vasc. Biol. 35, 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amano T., Matsubara T., Uetani T., Kato M., Kato B., Yoshida T., Harada K., Kumagai S., Kunimura A., Shinbo Y., Kitagawa K., Ishii H., Murohara T. (2011) Impact of omega-3 polyunsaturated fatty acids on coronary plaque instability: an integrated backscatter intravascular ultrasound study. Atherosclerosis 218, 110–116 [DOI] [PubMed] [Google Scholar]
- 38.Casula M., Soranna D., Catapano A. L., Corrao G. (2013) Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, double blind, placebo controlled trials. Atheroscler. Suppl. 14, 243–251 [DOI] [PubMed] [Google Scholar]
- 39.Mozaffarian D., Lemaitre R. N., King I. B., Song X., Huang H., Sacks F. M., Rimm E. B., Wang M., Siscovick D. S. (2013) Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann. Intern. Med. 158, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Oliveira Otto M. C., Wu J. H., Baylin A., Vaidya D., Rich S. S., Tsai M. Y., Jacobs D. R. Jr., Mozaffarian D. (2013) Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2, e000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosch J., Gerstein H. C., Dagenais G. R., Díaz R., Dyal L., Jung H., Maggiono A. P., Probstfield J., Ramachandran A., Riddle M. C., Rydén L. E., Yusuf S.; ORIGIN Trial Investigators (2012) n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 367, 309–318 [DOI] [PubMed] [Google Scholar]
- 42.Schwingshackl L., Christoph M., Hoffmann G. (2015) Effects of olive oil on markers of inflammation and endothelial function–a systematic review and meta-analysis. Nutrients 7, 7651–7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scoditti E., Calabriso N., Massaro M., Pellegrino M., Storelli C., Martines G., De Caterina R., Carluccio M. A. (2012) Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 527, 81–89 [DOI] [PubMed] [Google Scholar]
- 44.Covas M. I., Nyyssönen K., Poulsen H. E., Kaikkonen J., Zunft H.-J. F., Kiesewetter H., Gaddi A., de la Torre R., Mursu J., Bäumler H., Nascetti S., Salonen J. T., Fitó M., Virtanen J., Marrugat J.; EUROLIVE Study Group (2006) The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann. Intern. Med. 145, 333–341 [DOI] [PubMed] [Google Scholar]
- 45.Calder P. C. (2006) n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 83(6, Suppl)1505S–1519S [DOI] [PubMed] [Google Scholar]
- 46.Yacoubian S., Serhan C. N. (2007) New endogenous anti-inflammatory and proresolving lipid mediators: implications for rheumatic diseases. Nat. Clin. Pract. Rheumatol. 3, 570–579, quiz 1, 589 [DOI] [PubMed] [Google Scholar]
- 47.Cleland L. G., Caughey G. E., James M. J., Proudman S. M. (2006) Reduction of cardiovascular risk factors with longterm fish oil treatment in early rheumatoid arthritis. J. Rheumatol. 33, 1973–1979 [PubMed] [Google Scholar]
- 48.Lee Y. H., Bae S. C., Song G. G. (2012) Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch. Med. Res. 43, 356–362 [DOI] [PubMed] [Google Scholar]
- 49.Leeb B. F., Sautner J., Andel I., Rintelen B. (2006) Intravenous application of omega-3 fatty acids in patients with active rheumatoid arthritis. The ORA-1 trial. An open pilot study. Lipids 41, 29–34 [DOI] [PubMed] [Google Scholar]
- 50.James M. J., Proudman S. M., Cleland L. G. (2003) Dietary n-3 fats as adjunctive therapy in a prototypic inflammatory disease: issues and obstacles for use in rheumatoid arthritis. Prostaglandins Leukot. Essent. Fatty Acids 68, 399–405 [DOI] [PubMed] [Google Scholar]