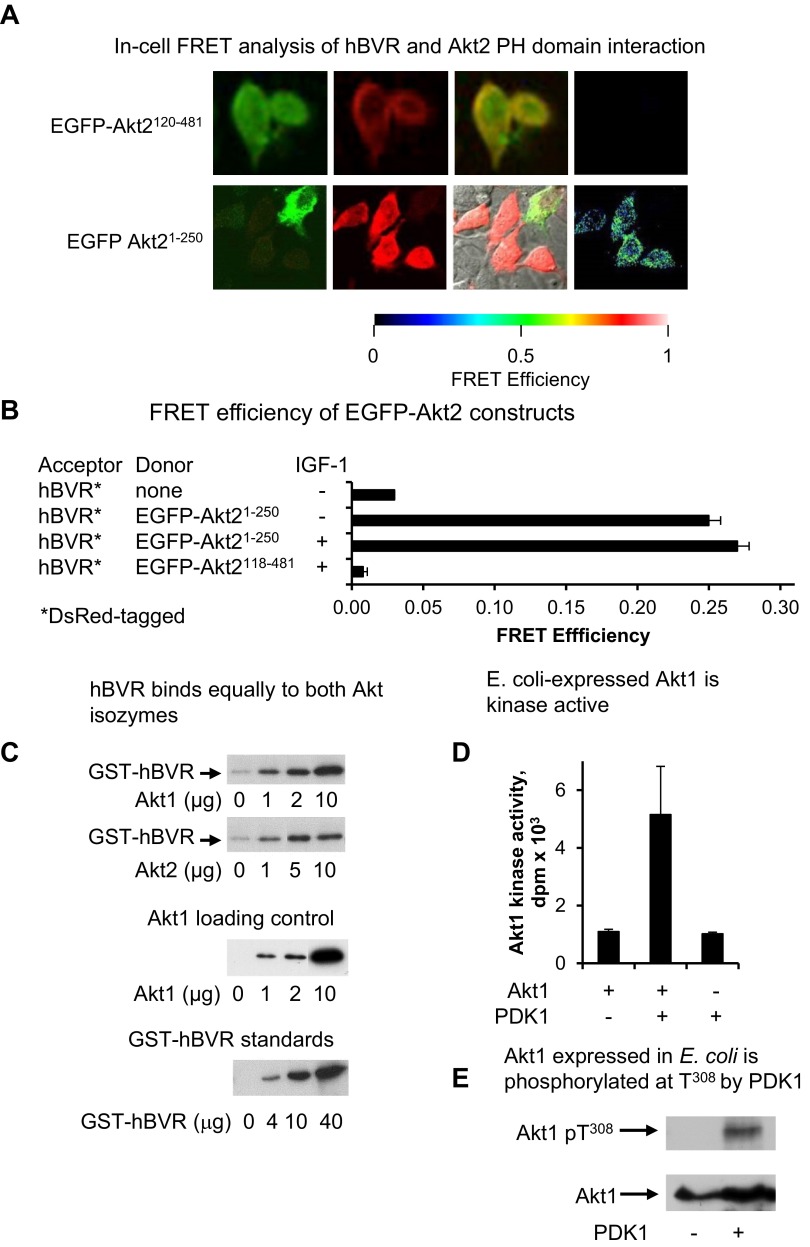
Figure 3.
A) In-cell FRET analysis of hBVR and Akt2 PH domain interaction. Cells cotransfected with pEGFP-Akt2 constructs and pDsRed2-hBVR were examined by FRET as in Fig. 2A. B) FRET efficiency was calculated as in Fig. 2B; efficiency for Akt21–250 construct was measured before and after IGF-1 treatment. C) hBVR does not display preferential affinity for Akt enzymes. His6-tagged Akts, expressed in E. coli and bound to Ni-NTA agarose, were incubated with GST-BVR. After washing, proteins bound on beads were analyzed by gel electrophoresis and Western blot test using anti-GST antibodies. A series of loading controls for GST-BVR was also included on blot to allow approximate quantification of GST-hBVR bound to Akt. The blot was subsequently stripped and probed with anti-Akt1 antibodies to verify input Akt1 levels. D) E. coli–expressed Akt1 is kinase active. his6-tagged Akt1 bound to Ni-NTA agarose was equilibrated in PDK1 kinase buffer and treated with PDK1. PDK1 was then washed from agarose beads, immobilized protein was equilibrated in Akt kinase buffer, and kinase activity was measured with Aktide substrate. E) Akt1 expressed in E. coli is phosphorylated at T308 by PDK1. PDK1 kinase reaction products from panel D were examined for T308 phosphorylation by Western blot test.