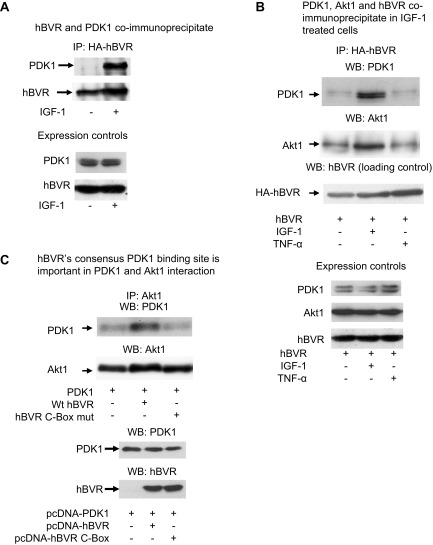
Figure 7.
A) Coimmunoprecipitation of PDK1 and hBVR. Cells were cotransfected with PDK1 expression plasmid and pcDNA-HA-hBVR. Lysates were prepared from cells with or without stimulation with 40 ng/ml IGF-1 and immunoprecipitated with anti-HA antibody. Immunoprecipitates were analyzed by Western blot test; blot was sequentially probed with antibodies to PDK1 and hBVR. Expression of PDK1 and hBVR in lysates was verified by Western blot test. B) PDK1, Akt1, and hBVR coimmunoprecipitate in IGF-1 stimulated cells. Lysates prepared from cells cotransfected with pcDNA-HA-hBVR and pcDNA-Akt1 and stimulated with 40 ng/ml IGF-1 or 50 ng/ml TNF-α were immunoprecipitated with anti-HA antibodies. Immunoprecipitates were analyzed by Western blot test, which was sequentially probed with antibodies to PDK1, Akt1, and hBVR. Overexpression of Akt1 and HA-hBVR, and presence of PDK1 were verified by Western blot test of cell lysates. C) hBVR consensus PDK1 binding site is important in PDK1 and Akt1 interaction. Cells were cotransfected with PDK1 expression plasmid with or without pcDNA-HA-hBVR or pcDNA-HA-hBVR C-Box (RFGFPAFS to RVGAPAVS) mutant. Immunoprecipitates obtained with Akt1 antibody were analyzed for PDK1 as in (B); equality of loading was confirmed by probing blot with anti-Akt1 antibody. Overexpression of proteins was verified by Western blot test of cell lysates.