Abstract
Objective:
To evaluate the feasibility, safety, and tolerability of noninvasive vagus nerve stimulation (nVNS) for the prevention of chronic migraine (CM) attacks.
Methods:
In this first prospective, multicenter, double-blind, sham-controlled pilot study of nVNS in CM prophylaxis, adults with CM (≥15 headache d/mo) entered the baseline phase (1 month) and were subsequently randomized to nVNS or sham treatment (2 months) before receiving open-label nVNS treatment (6 months). The primary endpoints were safety and tolerability. Efficacy endpoints in the intent-to-treat population included change in the number of headache days per 28 days and acute medication use.
Results:
Fifty-nine participants (mean age, 39.2 years; mean headache frequency, 21.5 d/mo) were enrolled. During the randomized phase, tolerability was similar for nVNS (n = 30) and sham treatment (n = 29). Most adverse events were mild/moderate and transient. Mean changes in the number of headache days were −1.4 (nVNS) and −0.2 (sham) (Δ = 1.2; p = 0.56). Twenty-seven participants completed the open-label phase. For the 15 completers initially assigned to nVNS, the mean change from baseline in headache days after 8 months of treatment was −7.9 (95% confidence interval −11.9 to −3.8; p < 0.01).
Conclusions:
Therapy with nVNS was well-tolerated with no safety issues. Persistent prophylactic use may reduce the number of headache days in CM; larger sham-controlled studies are needed.
ClinicalTrials.gov identifier:
Classification of evidence:
This study provides Class II evidence that for patients with CM, nVNS is safe, is well-tolerated, and did not significantly change the number of headache days. This pilot study lacked the precision to exclude important safety issues or benefits of nVNS.
Migraine is a disabling neurologic headache disorder with symptoms including nausea and sensitivity to light/sound.1 Compared with episodic migraine (headache occurring <15 d/mo), chronic migraine (CM; headache occurring ≥15 d/mo) leads to greater disability and lower productivity.1,2 Although preventive β-blockers, tricyclic antidepressants, and anticonvulsants are used off-label in CM,2,3 onabotulinumtoxinA is the only approved prophylactic CM medication.4,5
Neuromodulation using implanted vagus nerve stimulation (VNS) devices has demonstrated efficacy for the treatment of epilepsy6 and depression7 and potential efficacy for migraine prophylaxis.8,9 Case reports and small studies of patients who received implanted VNS devices for epilepsy showed reductions in migraine attack frequency and severity.8–12 Epilepsy and depression studies of VNS suggest that efficacy improves with time on therapy6,7; however, this association has not been investigated in migraine prophylaxis.
Despite the potential benefits of implanted VNS therapy, the high risks and costs of surgical implantation have hindered its clinical evaluation. A patient-controlled, handheld, noninvasive VNS (nVNS) device (gammaCore®; electroCore, LLC, Basking Ridge, NJ) has been CE-marked for the treatment of primary headache disorders (including migraine). Here we describe preliminary experience with nVNS for CM prophylaxis. These data were first reported at the 56th annual meeting of the American Headache Society (June 26–29, 2014; Los Angeles, CA).13
METHODS
Primary objective.
The objective of this study was to assess the feasibility, safety, and tolerability of nVNS; it was not powered to assess efficacy.
Standard protocol approvals and patient consents.
The protocol was approved by site-specific institutional review boards for 2 of the 6 participating sites and by the Biomedical Research Alliance of New York institutional review board for the remaining 4 sites. All participants provided signed informed consent before enrollment.
Study design.
This prospective pilot study of nVNS for CM prophylaxis was conducted at 6 US tertiary care headache centers between October 2012 and April 2014 (EVENT; ClinicalTrials.gov NCT01667250). The study comprised 3 consecutive phases: a 1-month baseline phase to collect pretreatment data and medical history; a 2-month, double-blind, randomized, sham-controlled phase, during which participants received prophylactic treatment with nVNS or a sham device; and a 6-month open-label phase, during which all participants received nVNS treatment. For the total 8-month treatment period, the end of the 2-month randomized phase was denoted as month 2, and the three 2-month evaluation points during the 6-month open-label phase were denoted as months 4, 6, and 8.
Study population.
Participants were aged 18–65 years and previously diagnosed with CM with/without aura according to the revised International Classification of Headache Disorders, second edition, criteria,14,15 had migraine onset before 50 years of age, and had ≥15 headache d/mo during the previous 3 months.
Key exclusion criteria were a history of aneurysm, intracranial hemorrhage, brain tumor, or head trauma; a lesion, dysesthesia, previous surgery, or abnormal anatomy at the treatment site; known or suspected cardiovascular disease; uncontrolled hypertension; abnormal ECG results; recent myocardial infarction; an implanted electrical/neurostimulator device; a metallic implant/metal cervical spine hardware near the stimulation site; previous surgery for migraine prevention; onabotulinumtoxinA injections for migraine prevention during the previous 6 months; and prophylactic migraine medication during the previous 30 days. Modifications in prophylactic medication type/dose for indications other than CM that could interfere with the study were not permitted.
Randomization and blinding.
An independent statistician generated a randomization schedule to assign participants 1:1 (variable block design stratified by study center) to prophylactic treatment with nVNS or sham treatment. The study sponsor (electroCore, LLC) prelabeled the devices according to each site's randomization scheme; a third-party distributor provided the devices to study sites. An unblinded trainer provided participants with the devices and instructions on device features, proper use, and treatment schedules. Participants, investigators, and study coordinators were blinded to treatment assignment during the randomized phase.
Interventions.
The nVNS device produced a proprietary electrical signal that delivered a low voltage (peak, 24 V) and a maximum output current of 60 mA. Users adjusted the stimulation amplitude within a preset range. Two stainless steel contact surfaces coated with a conductive gel enabled delivery of stimulations to the neck in the vicinity of the vagus nerve (figure e-1 on the Neurology® Web site at Neurology.org). The sham device was identical in appearance, weight, visual and audible feedback, and user application and control but did not deliver electrical stimulations. Each treatment consisted of two 2-minute self-administered stimulations delivered 5–10 minutes apart to the right side of the neck at 3 prespecified times every day: (1) within 1 hour of awakening; (2) 6–8 hours after the first treatment; and (3) 6–8 hours after the second treatment. Acute headache medication use was permitted throughout the study.
Assessments and endpoints.
Participants used diaries to record safety and tolerability (primary endpoints), efficacy, and satisfaction data. Investigators categorized the onset, type, severity (mild, moderate, severe), and frequency of adverse events (AEs) according to treatment relatedness. Serious AEs (SAEs) were defined by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use: Guidance for Good Clinical Practice.
The number of reported headache days per month was normalized to the number of headache days per 28 days, which was the primary efficacy measure. A headache day was defined as any day on which a participant recorded a headache. The mean change from baseline in the number of headache days was evaluated at the end of the randomized phase (month 2) and through the end of the open-label phase (at 4, 6, and 8 months of treatment). Post hoc efficacy analyses assessed the effect of treatment duration on the number of headache days and determined the percent treatment response, defined as the proportion of participants who demonstrated ≥50% reduction from baseline in the number of headache days.
The rate of patient-reported acute medication use and treatment adherence, satisfaction, and ease of use were evaluated throughout both phases. Treatment adherence ([actual number of administered treatments]/[total number of scheduled treatments] × 100) was calculated as the average daily adherence. Treatment satisfaction was assessed on a 5-point scale (extremely satisfied to not at all satisfied). Ease of use was rated on a 4-point scale (very easy to very difficult). One week into the randomized phase and at its end, study blinding effectiveness questionnaires were completed.
Statistical methods.
No formal sample size calculations were performed; the sample size was selected to facilitate initial assessment of feasibility and tolerability in a clinically relevant number of participants. All analyses were conducted on the intent-to-treat (ITT) population, which included all participants who were randomly assigned to treatment and provided data for each outcome. Missing data were imputed using last observation carried forward (LOCF). To assess the effect of protocol deviation and discontinuations, sensitivity analyses were performed on the per-protocol (PP) population, which included only participants who completed each phase with no major protocol violations. Pooled participants from both treatment groups in the PP population (i.e., the PP completer population) were stratified and analyzed by the total duration of nVNS treatment completed throughout the study (2-, 4-, 6-, or 8-month completers). Specifically, 2-month completers comprised participants in the nVNS group who completed the 2-month randomized phase and participants in the sham group (controls) who completed 2 months of open-label nVNS treatment.
There were no formal a priori statistical analyses; exploratory post hoc analyses were conducted to determine the effect of nVNS treatment duration on the mean change in number of headache days and to compare treatment responses for nVNS and sham. Categorical variables were compared using the Fisher exact test (if ≥1 cell had an expected frequency ≤5) or χ2 analyses. Continuous variables were compared using the Student t test and the Wilcoxon rank sum test for normal and non-normal distributions, respectively. Blinding questionnaire results were analyzed using the Bang index16 and corresponding 95% confidence intervals (CIs).
RESULTS
Participants.
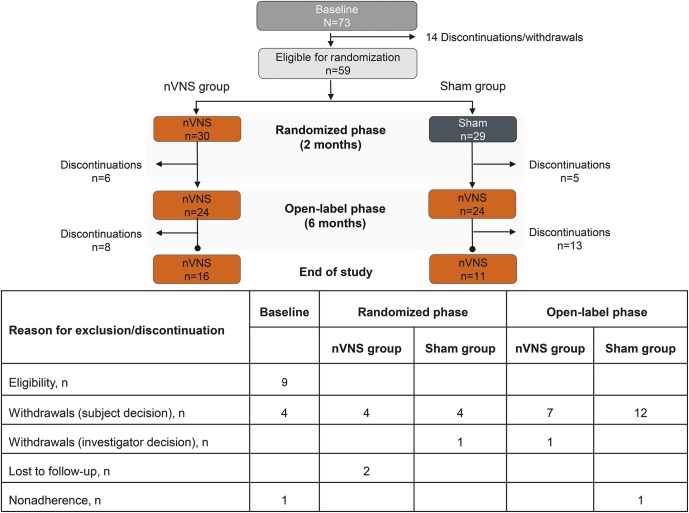
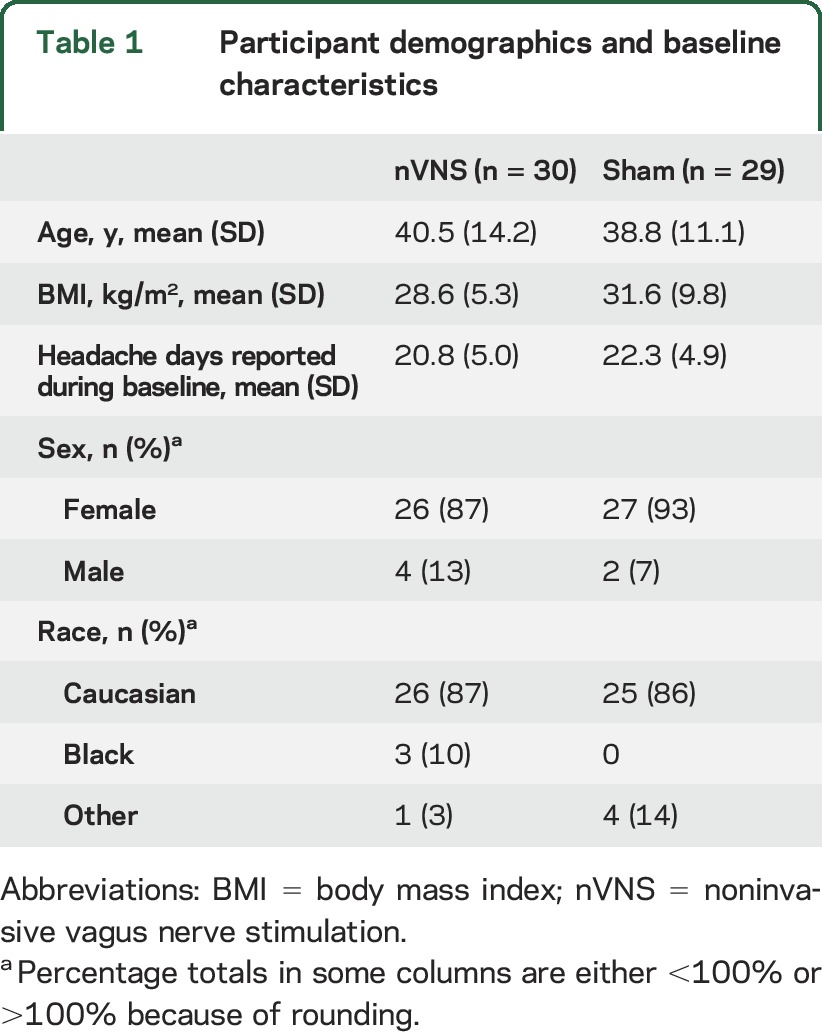
Fifty-nine of the 73 participants in the baseline phase were eligible for randomization and constituted the ITT population (nVNS, n = 30; sham, n = 29) (figure 1). A total of 51 participants (nVNS, n = 26; sham, n = 25) from the ITT population and 49 participants (nVNS, n = 26; sham, n = 23) from the PP population (i.e., no violations) completed the randomized phase, and 48 participants (nVNS, n = 24; sham, n = 24) from the ITT population and 47 participants (nVNS, n = 24; sham, n = 23) from the PP population continued into the open-label phase. Twenty-seven participants completed the study (nVNS, n = 16; sham, n = 11). Safety analyses were performed on all 59 participants from the ITT population. Demographic and baseline characteristics were similar to those reported in other migraine studies (table 1).17 Participants (mean age, 39 years; mean headache frequency, >20 d/mo) were predominantly Caucasian women.
Figure 1. Participant disposition.
nVNS = noninvasive vagus nerve stimulation.
Table 1.
Participant demographics and baseline characteristics
Safety and tolerability.
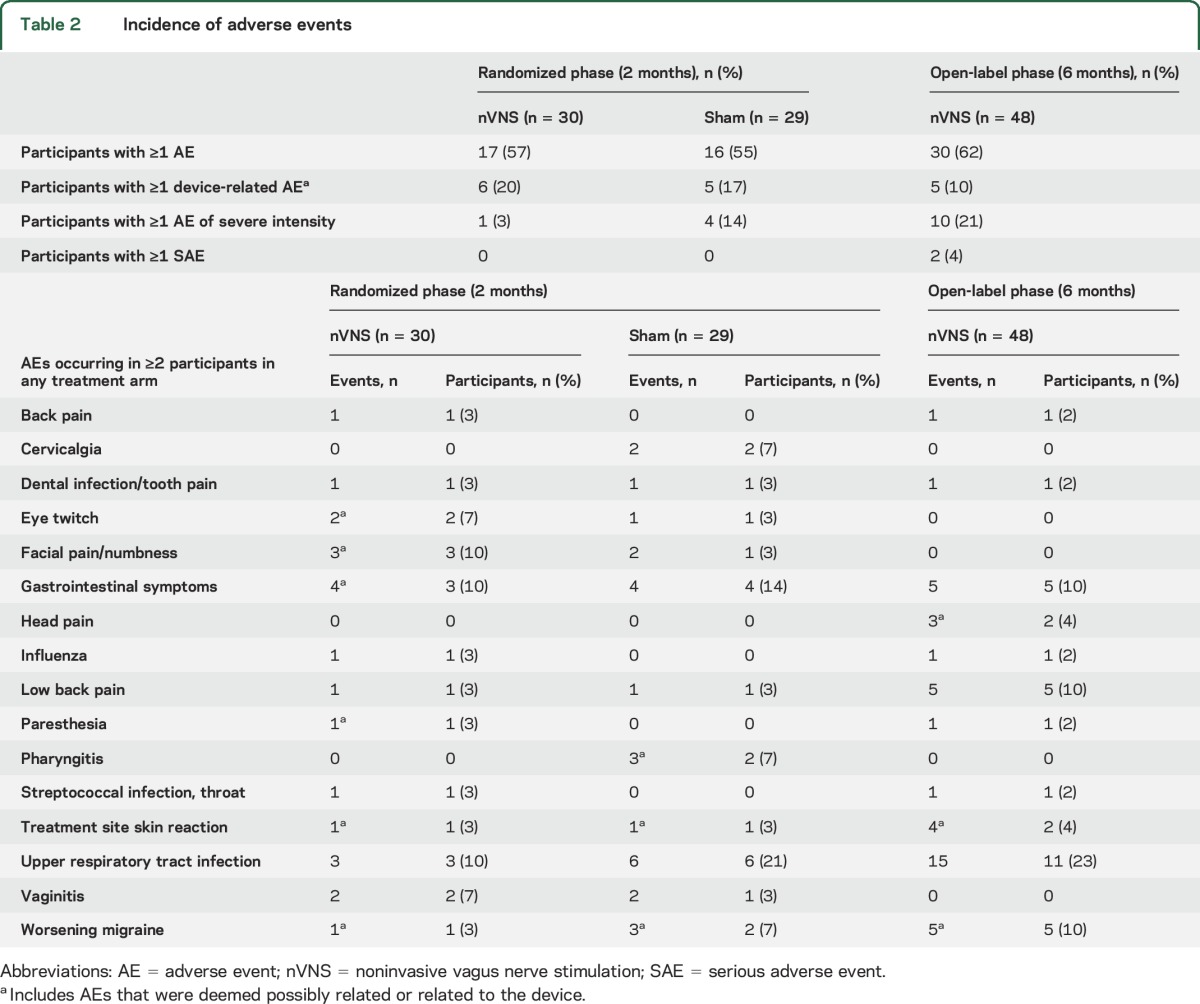
The tolerability profile of nVNS was satisfactory and generally similar to that of sham treatment (table 2). Most AEs were mild or moderate and transient. The most commonly reported AEs were upper respiratory tract infections and gastrointestinal symptoms. During the randomized phase, 6 nVNS-treated participants reported 12 AEs that were related or possibly related to the device, whereas 5 controls reported 8 such AEs. No SAEs occurred during the randomized phase. During the open-label phase, 5 participants reported 8 AEs that were related or possibly related to the device. Two participants reported SAEs during the open-label phase (i.e., appendicitis and worsening headache); both were unrelated to the device. No discontinuations due to device-related AEs occurred.
Table 2.
Incidence of adverse events
Number of headache days.
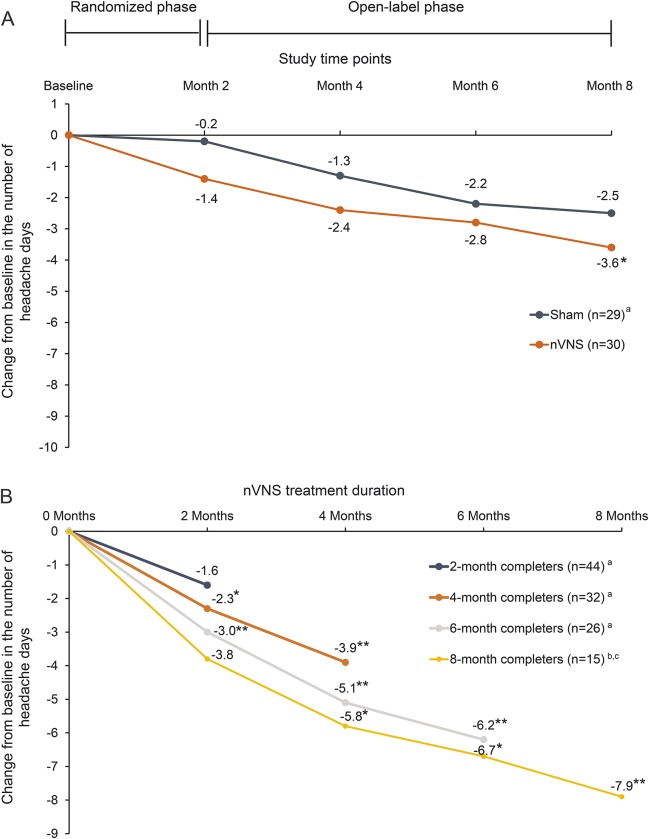
The mean number of headache days in the nVNS group (n = 30) was 20.8 (95% CI 18.9–22.6) at baseline and 19.4 (95% CI 16.6–22.1) at month 2 (end of the randomized phase), representing a mean change of −1.4 (95% CI −3.7 to 0.77; p = 0.44) (figure 2A). The mean number of headache days in controls (n = 29) was 22.3 (95% CI 20.4–24.1) at baseline and 22.0 (95% CI 19.5–24.6) at month 2, demonstrating a mean change of −0.2 (95% CI −1.5 to 1.1; p = 0.72) (figure 2A). The mean change from baseline was not statistically different between groups (p = 0.56). The difference between groups was slightly more pronounced in the PP population, with mean changes of −2.0 (95% CI −4.4 to 0.4) for nVNS and −0.1 (95% CI −1.6 to 1.4) for sham (p = 0.35).
Figure 2. Effects of noninvasive vagus nerve stimulation (nVNS) on the change in number of headache days per 28 days.
(A) Mean change in headache days per 28 days from baseline through the open-label phase (intent-to-treat population). Imputation for missing data was performed using the last observation carried forward. aReceived open-label nVNS after month 2. *p < 0.05 vs baseline. (B) Mean change in headache days per 28 days from baseline through the duration of nVNS treatment (per-protocol completer population). aThe 2-, 4-, and 6-month completers were from the 59 participants initially randomized to either nVNS or sham treatment. bThe 8-month completers were from the 30 participants initially randomized to nVNS treatment. cOne participant who completed 8 months of nVNS initiated gabapentin treatment during the study and was therefore excluded from 8-month completer analyses. *p < 0.05 vs baseline. **p < 0.01 vs baseline.
Persistent prophylactic nVNS use was associated with continued reductions in the number of headache days. After the open-label phase, participants initially assigned to nVNS had a mean of 17.2 (95% CI 13.8–20.5) headache days and a mean change from baseline of −3.6 (95% CI −6.3 to −0.87; p = 0.02) after 8 months of treatment (figure 2A). Participants initially randomized to sham treatment had a mean of 19.7 (95% CI 16.5–23.0) headache days and a mean change from baseline of −2.5 (95% CI −5.0 to −0.04; p = 0.06) after 6 months of nVNS (figure 2A). To investigate the effect of treatment persistence, mean changes in the number of headache days were analyzed without LOCF imputation. More pronounced and clinically meaningful reductions from baseline at months 4, 6, and 8 (−3.7, −6.1, and −8.0 headache days, respectively) were observed in the nVNS group (month 4, p < 0.05; months 6 and 8, p < 0.01). The mean change of −6.0 headache days for controls reached statistical significance at month 8 (p < 0.05).
In the PP completer population, longer duration was associated with greater reductions in headache days from study baseline (nVNS) and from month 2 (sham) (figure 2B). These mean reductions were 1.6 (95% CI 0.1–3.1; p = 0.06) for 2-month completers (n = 44), 3.9 (95% CI 1.4–6.3; p = 0.004) for 4-month completers (n = 32), 6.2 (95% CI 3.2–9.3; p < 0.0001) for 6-month completers (n = 26), and 7.9 (95% CI 11.9–3.8; p = 0.0009) for 8-month completers (n = 15) (figure 2B). In contrast with 2-month completers, 4-, 6-, and 8-month completers each experienced significant reductions at 2-, 4-, 6-, and 8-month timepoints except for the 8-month completers at the 2-month timepoint (p = 0.06) (figure 2B).
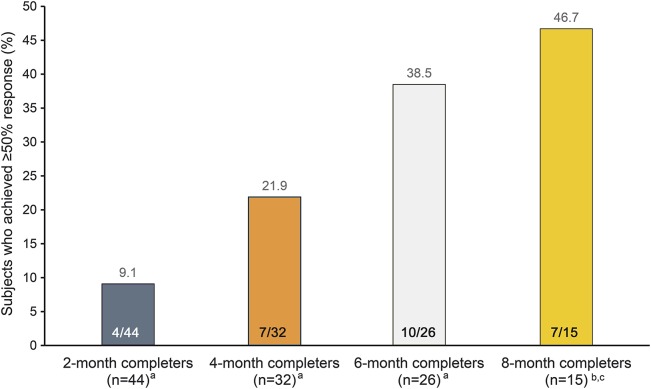
Treatment response.
At month 2, 10.0% (3/30) of participants from the nVNS group had a ≥50% response, and 3.3% (1/30) experienced a ≥75% response. No controls experienced a ≥50% response. In the PP population, 11.5% (3/26) of participants from the nVNS group had a ≥50% response; 3.8% (1/26) experienced a ≥75% response. No controls experienced a ≥50% response. In the PP completer populations, the proportion of participants who achieved a ≥50% response increased with longer duration of treatment throughout the open-label phase (figure 3).
Figure 3. Participants who achieved a ≥50% treatment response with noninvasive vagus nerve stimulation (nVNS) (per-protocol completer population).
aThe 2-, 4-, and 6-month completers were from the 59 participants initially randomized to either nVNS or sham treatment. bThe 8-month completers were from the 30 participants initially randomized to nVNS treatment. cOne participant who completed 8 months of nVNS initiated gabapentin treatment during the study and was therefore excluded from 8-month completer analyses.
Acute medication use.
Rates of acute medication use were comparable between groups and remained stable from baseline (89.8%) through the open-label phase (81.5%).
Treatment adherence, satisfaction, and ease of use.
Mean treatment adherence for both groups was ≥95% during the randomized phase and ≥92% during the open-label phase. At month 2, 58.3% (14/24) of nVNS-treated participants and 41.7% (10/24) of sham-treated participants were at least a little satisfied with treatment (p = 0.25; χ2 test). Among participants who completed the open-label phase, 88.5% (23/26) were satisfied with treatment. Most participants found the device somewhat easy or very easy to use (nVNS, 20/24; 83.3%; sham, 22/24; 91.7%).
Blinding evaluation.
At the beginning of the randomized phase, a similar number of participants in each arm (nVNS, 37.9% [11/29]; sham, 39.3% [11/28]) correctly identified their treatment. The groups had similar Bang index values (nVNS, 0.31 [95% CI 0.15–0.47]; sham, 0.32 [95% CI 0.15–0.49]), each indicating that blinding was not achieved. At month 2, Bang index values of 0.04 (95% CI 0.0–0.25) for nVNS and 0.54 (95% CI 0.31–0.77) for sham suggest blinding was achieved in the nVNS group but not in controls.
DISCUSSION
We demonstrated that nVNS for CM prevention was well-tolerated and identified no safety issues; preliminary efficacy results showed that the reduction in headache days during the 2-month randomized phase among participants receiving nVNS compared with controls was not significant. We assessed whether longer treatment duration was associated with clinically meaningful results, as previously reported in a study of neuromodulation in CM.18 Participants originally randomized to nVNS and who continued open-label treatment for 6 months had a significant reduction from baseline in the number of headache days. Longer treatment durations were associated with greater reductions in number of headache days and higher ≥50% response rates, recognizing that the 27 participants who completed the open-label phase of the trial and received longer treatment (16 and 11 of whom were initially randomized to nVNS and sham, respectively) may have been self-selected.
Among study completers, the ≥50% response rate increased with time on treatment, which supports the slow accrual of clinical benefits over time reported in VNS studies of epilepsy and depression.6,7 Loss to follow-up cannot explain this result; however, regression toward the mean may be a confounding factor. The association between longer nVNS treatment duration and a gradual reduction in headache days suggests that participants who discontinued the study might have benefited from continued treatment, consistent with other findings in the literature.6,7,19 Conversely, participants who continued treatment may represent self-identified responders for whom the device is effective.
Study limitations included the small sample size, blinding challenges, and high discontinuation rate. Blinding in device studies is challenging, especially in comparison with drug studies.20 Our sham device was identical to the nVNS device but did not deliver an active signal. A sham device should mimic the functionality and sensation of active treatment without producing treatment effects or device-related AEs. Missing data and high discontinuation rates occurring disproportionately across treatment groups can affect study outcomes. In this study, discontinuation rates were higher in controls than in the nVNS group; however, no discontinuations stemmed from device-related AEs.
The emergence of noninvasive neuromodulation devices has sparked interest in their application in migraine therapy. Two short-term, double-blind, randomized, controlled trials have examined prophylactic therapy for migraine using noninvasive neuromodulation devices.21,22 One multicenter sham-controlled study reported a significant reduction in the mean number of headache days following transcutaneous supraorbital stimulation for 3 months in participants with migraine; however, the proportion of participants with CM was unknown.21 A single-center controlled study of prophylactic transcutaneous auricular VNS therapy in CM showed that the reduction in number of headache days was significantly greater at a stimulation frequency of 1 Hz than at 25 Hz; however, no sham arm was available for comparison.22 EVENT is the first multicenter, double-blind, randomized, sham-controlled trial to demonstrate the long-term (8-month) tolerability and preliminarily evaluate the efficacy of prophylactic nVNS therapy in a clinically defined CM population. Stimulation parameters used in EVENT (i.e., 2-minute stimulations administered 3 times per day on the right side of the neck) were based on both previous open-label clinical studies in migraine and cluster headache23,24 and preclinical models of migraine and airways disease,25–27 but bilateral stimulations are currently being used in ongoing studies, which may affect efficacy results observed in these and future trials. Findings from the animal migraine models suggest that nVNS may exert beneficial effects via the suppression of glutamate levels and cortical spreading depression, a key factor in migraine pathophysiology.25,27 Prophylactic nVNS has been shown to be clinically beneficial19 and cost-effective28 in chronic cluster headache. Findings from EVENT expand the evidence regarding the potential role of noninvasive neuromodulation in headache therapy. Given the need for novel prophylactic therapies for CM and the high cost of the currently approved medication,29,30 nVNS may serve as a well-tolerated and potentially cost-effective alternative for patients with CM.
nVNS is a highly feasible, well-tolerated, and convenient therapy. Although self-selection bias is associated with the long-term findings, the continued reduction in headache days over the 6-month open-label phase suggests that nVNS may offer a clinical benefit to patients with CM. Longer-term use of nVNS in treatment responders would be reflective of clinical practice.31 On the basis of the lack of a plateau in effects seen during the open-label phase, larger studies using modified stimulation parameters and longer open-label periods may validate the use of nVNS in migraine therapy; a study with a 9-month open-label period is currently planned.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the investigators and study sites (coinvestigator appendix at Neurology.org); the EVENT study investigators thank the nurses and study coordinators; professional writing and editorial support (i.e., technical editing, copyediting, preparation of tables and figures, and clerical assistance) for this manuscript was provided by MedLogix Communications, LLC (Schaumburg, IL) under the direction of the authors.
GLOSSARY
- AE
adverse event
- CI
confidence interval
- CM
chronic migraine
- ITT
intent-to-treat
- LOCF
last observation carried forward
- nVNS
noninvasive vagus nerve stimulation
- PP
per-protocol
- SAE
serious adverse event
- VNS
vagus nerve stimulation
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: EVENT Study Group, Anne H. Calhoun, Roger K. Cady, John Dexter, Stephen D. Silberstein, William Young, Michael Marmura, Stephanie Nahas-Geiger, Arnaldo Da Silva, Joel Saper, James Weintraub, Alicia Prestegaard, Erik Sinka, Brian Grosberg, Sarah Vollbracht, Shirin Issa, Richard Lipton, Kathleen Mullin, Jelena Pavlovic, Matthew Robbins, Peter Goadsby, Amy Gelfand, and Michael Eller
AUTHOR CONTRIBUTIONS
This study was sponsored by electroCore, LLC. Professional writing and editorial support (i.e., technical editing, copyediting, preparation of tables and figures, and clerical assistance) from MedLogix Communications, LLC, funded by electroCore, LLC, was based on author direction throughout draft development and revisions. Data analysis support from NAMSA was funded by electroCore, LLC. Dr. Stephen D. Silberstein, Dr. Peter J. Goadsby, Eric J. Liebler, and Kristy A. Simmons contributed to the EVENT study design and provided detailed input into the development of the manuscript. Stefanie Dorlas is responsible for drafting/revising the manuscript for content, including medical writing for content. All primary investigators were involved in participant recruitment and treatment for the EVENT study. All authors participated in data collection, interpretation, and validation. Kristy A. Simmons, Eric J. Liebler, and NAMSA were involved in data analysis. All authors reviewed, critiqued, and contributed to revision of the manuscript content and provided approval of the final manuscript draft to be submitted to Neurology®. The corresponding author, Dr. Stephen D. Silberstein, had full access to all the study data and had final responsibility for the decision to submit the manuscript for publication.
STUDY FUNDING
This study was sponsored by electroCore, LLC.
DISCLOSURE
S. Silberstein has received honoraria as a consultant and/or advisory panel member from Alder Biopharmaceuticals; Allergan, Inc.; Amgen Inc.; Avanir Pharmaceuticals, Inc.; Depomed, Inc.; Dr. Reddy's Laboratories Ltd.; electroCore, LLC; eNeura, Inc.; Ipsen Biopharmaceuticals; Medscape, LLC; Medtronic, Inc.; Mitsubishi Tanabe Pharma America, Inc.; NINDS; St. Jude Medical; Supernus Pharmaceuticals, Inc.; Teva Pharmaceuticals; and Trigemina, Inc. A. Calhoun has participated as an advisory board member for Allergan, Inc.; Depomed, Inc.; and Teva Pharmaceuticals. Dr. Calhoun has received honoraria from and participated as a speaker's bureau member for Depomed, Inc.; Merck & Co., Inc.; and Teva Pharmaceuticals. She has also received research support from Autonomic Technologies, Inc.; electroCore, LLC; and Scion NeuroStim LLC. B. Grosberg reports no disclosures relevant to the manuscript. R. Lipton receives research support from the National Headache Foundation and the NIH: PO1AG003949 (program director), RO1AG025119 (investigator), RO1AG022374-06A2 (investigator), RO1AG034119 (investigator), and RO1AG12101 (investigator); serves on the editorial board of Neurology and as senior advisor to Headache; has reviewed for the National Institute on Aging and NINDS; holds stock options in eNeura, Inc.; and serves as a consultant or advisory board member or has received honoraria from Alder Biopharmaceuticals; Allergan, Inc.; American Headache Society; Amgen, Inc.; Autonomic Technologies, Inc.; Avanir Pharmaceuticals, Inc.; Boston Scientific Corporation; CoLucid Pharmaceuticals, Inc.; Dr. Reddy's Laboratories Ltd.; electroCore, LLC; Eli Lilly and Company; eNeura Therapeutics; Informa; Merck & Co., Inc.; Teva Pharmaceuticals; and Vedanta Biosciences. R. Cady has participated as a consultant, advisory board member, and speaker's bureau member for Allergan, Inc., Depomed, Inc., and Teva Pharmaceutical Industries Ltd. Dr. Cady has also participated as an advisory board member for Aerocrine Inc.; Amgen, Inc.; Autonomic Technologies, Inc.; Avanir Pharmaceuticals, Inc.; Boston Scientific Corporation; Dr. Reddy's Laboratories Ltd.; electroCore, LLC; Novartis; and Suda Ltd. He has served as a consultant and speaker's bureau member for Impax Pharmaceuticals; as a consultant for Becker Pharmaceuticals Consulting and Zosano Pharma Corporation; and as a speaker's bureau member for Merck & Co., Inc. Dr. Cady has also received research support from Alder Biopharmaceuticals; Allergan, Inc.; Amgen Inc.; AstraZeneca; Autonomic Technologies, Inc.; Boston Scientific Corporation; Capnia; Daiichi-Sankyo; Dr. Reddy's Laboratories Ltd.; electroCore, LLC; Eli Lilly and Company; Labrys Biologics, Inc.; OptiNose; Pharmalyte Solutions, LLC; Questcor Pharmaceuticals, Inc.; Tian Medical; and Vivid Pharmaceuticals. P. Goadsby has received honoraria from Ajinomoto Pharmaceuticals Co., Ltd.; Akita Pharmaceuticals, Ltd.; Amgen Inc.; Alder Biopharmaceuticals; Allergan, Inc.; Autonomic Technologies, Inc.; Avanir Pharmaceuticals, Inc.; Cipla Ltd.; CoLucid Pharmaceuticals, Inc.; Dr. Reddy's Laboratories Ltd.; Eli Lilly and Company; eNeura, Inc.; W.L. Gore & Associates, Inc.; Heptares Therapeutics; Ethicon, Inc.; NEJM Journal Watch; NuPathe Inc.; Pfizer, Inc.; Promius Pharma, LLC; Teva Pharmaceutical Industries Ltd.; UptoDate®.com (outside the submitted work); Wells Fargo; and Zosano Pharma Corporation. He has also received honoraria for medicolegal work in headache and grants from Allergan, Inc., and Amgen Inc. and has a pending patent for magnetic stimulation for headache. K. Simmons was an employee of electroCore, LLC at the time of study completion. C. Mullin is an employee of NAMSA. E. Liebler is an employee of electroCore, LLC and receives stock ownership. Stefanie Dorlas is employed by MedLogix Communications, LLC, as a medical writer. J. Saper has received research grants from Achelios Therapeutics, Inc.; Alder Biopharmaceuticals; Allergan, Inc.; Amgen Inc.; Astellas Pharma Inc.; Autonomic Technologies, Inc.; Cerephex Corporation; Daiichi-Sankyo; Dr. Reddy's Laboratories Ltd.; GlaxoSmithKline; Labrys Biologics, Inc.; Lilly; Merck & Co., Inc.; Pfizer, Inc.; Scion NeuroStim LLC; Vanda Pharmaceuticals; and Winston Laboratories, Inc.; and has received honoraria as a consultant for Alder Biopharmaceuticals; Allergan, Inc.; Johnson & Johnson (Ethicon, Inc.); the Migraine Research Foundation; NuPathe Inc.; Purdue Pharma; Supernus Pharmaceuticals; Teva Pharmaceutical Industries Ltd.; and Tian Pharmaceutical Co. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 2.Schwedt TJ. Chronic migraine. BMJ 2014;348:g1416. [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ, Sprenger T. Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol 2010;9:285–298. [DOI] [PubMed] [Google Scholar]
- 4.Aurora SK, Dodick DW, Turkel CC, et al. . OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010;30:793–803. [DOI] [PubMed] [Google Scholar]
- 5.Diener HC, Dodick DW, Aurora SK, et al. . OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010;30:804–814. [DOI] [PubMed] [Google Scholar]
- 6.Murphy JV. Left vagal nerve stimulation in children with medically refractory epilepsy: The Pediatric VNS Study Group. J Pediatr 1999;134:563–566. [DOI] [PubMed] [Google Scholar]
- 7.Aaronson ST, Carpenter LL, Conway CR, et al. . Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul 2013;6:631–640. [DOI] [PubMed] [Google Scholar]
- 8.Lenaerts ME, Oommen KJ, Couch JR, Skaggs V. Can vagus nerve stimulation help migraine? Cephalalgia 2008;28:392–395. [DOI] [PubMed] [Google Scholar]
- 9.Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia 2005;25:82–86. [DOI] [PubMed] [Google Scholar]
- 10.Cecchini AP, Mea E, Tullo V, et al. . Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci 2009;30(suppl 1):S101–S104. [DOI] [PubMed] [Google Scholar]
- 11.Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. J Pain 2003;4:530–534. [DOI] [PubMed] [Google Scholar]
- 12.Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia 2002;22:482–484. [DOI] [PubMed] [Google Scholar]
- 13.Silberstein SD, Da Silva AN, Calhoun AH, et al. . Non-invasive vagus nerve stimulation for chronic migraine prevention in a prospective, randomized, sham-controlled pilot study (the EVENT study): report from the double-blind phase. Headache 2014;54:LBP19. [Google Scholar]
- 14.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- 15.Olesen J, Bousser MG, Diener HC, et al. . New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 2006;26:742–746. [DOI] [PubMed] [Google Scholar]
- 16.Bang H, Flaherty SP, Kolahi J, Park J. Blinding assessment in clinical trials: a review of statistical methods and a proposal of blinding assessment protocol. Clin Res Reg Aff 2010;27:42–51. [Google Scholar]
- 17.Silberstein SD, Lipton RB, Dodick DW. Operational diagnostic criteria for chronic migraine: expert opinion. Headache 2014;54:1258–1266. [DOI] [PubMed] [Google Scholar]
- 18.Dodick DW, Silberstein SD, Reed KL, et al. . Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 2015;35:344–358. [DOI] [PubMed] [Google Scholar]
- 19.Gaul C, Diener HC, Silver N, et al. . Non-invasive vagus nerve stimulation for prevention and acute treatment of chronic cluster headache (PREVA): a randomized controlled study. Cephalalgia 2016;36:534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broadbent HJ, van den Eynde F, Guillaume S, et al. . Blinding success of rTMS applied to the dorsolateral prefrontal cortex in randomised sham-controlled trials: a systematic review. World J Biol Psychiatry 2011;12:240–248. [DOI] [PubMed] [Google Scholar]
- 21.Schoenen J, Vandersmissen B, Jeangette S, et al. . Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology 2013;80:697–704. [DOI] [PubMed] [Google Scholar]
- 22.Straube A, Ellrich J, Eren O, Blum B, Ruscheweyh R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain 2015;16:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goadsby PJ, Grosberg BM, Mauskop A, Cady R, Simmons KA. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia 2014;34:986–993. [DOI] [PubMed] [Google Scholar]
- 24.Nesbitt AD, Marin JC, Tompkins E, Ruttledge MH, Goadsby PJ. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology 2015;84:1249–1253. [DOI] [PubMed] [Google Scholar]
- 25.Chen SP, Ay I, Lopes de Morais A, et al. . Vagus nerve stimulation inhibits cortical spreading depression. Pain 2016;157:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann TJ, Simon BJ, Zhang Y, Emala CW. Low voltage vagal nerve stimulation reduces bronchoconstriction in guinea pigs through catecholamine release. Neuromodulation 2012;15:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshinsky ML, Murphy AL, Hekierski H Jr, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain 2014;155:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaul C, Straube A, Reuter U, Morris J, Walker S, Diener HC. Cost-effectiveness analysis of non-invasive vagus nerve stimulation for the treatment of chronic cluster headache in Germany. Cephalalgia 2015;35:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botulinum toxin for chronic migraine. Med Lett Drugs Ther 2011;53:7–8. [PubMed] [Google Scholar]
- 30.Khalil M, Zafar HW, Quarshie V, Ahmed F. Prospective analysis of the use of onabotulinumtoxinA (BOTOX) in the treatment of chronic migraine: real-life data in 254 patients from Hull, U.K. J Headache Pain 2014;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moscato D, Moscato FR. A survey of patient perceptions of non-invasive vagus nerve stimulation (nVNS) therapy for acute migraine attacks. Cephalalgia 2015;35(suppl 6):PO016. Abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.