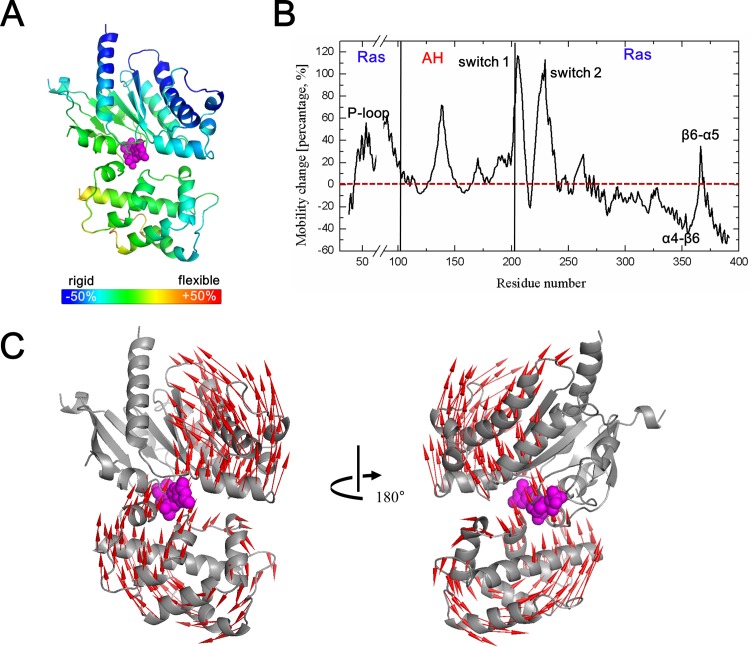
Fig 5. Dynamic features of the R-Gαβγ(GDP) complex.
(A and B) Change in the mean squared fluctuation of Gαs upon β2AR binding. Mobility changes of switches 1 and 2, P-loop, β6-α5 loop, α4-β6 loop, and α5 helix are marked here. Overall, the GαsRas domain loses its intrinsic flexibility due to direct binding to the receptor. (C) Shape of R-Gαβγ(GDP) in the fourth normal mode. The open motion corresponding to the conformational change direction in Gα is shown.