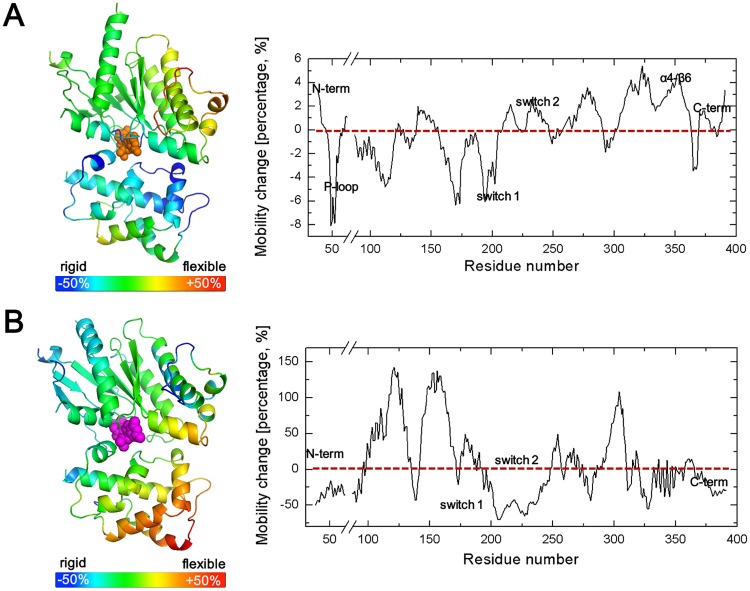
Fig 7. Role of GTP and its hydrolysis in Gαs mobility.
(A) Change in mean squared fluctuation of Gαs in R-Gαβγ(0) following the addition of GTP. Receptor binding regions (N terminus, C terminus, α4-β6 loop) and Gβγ binding regions (switch 2) became highly mobile for easy dissociation of bound structures. In contrast, the mean squared fluctuation values of nucleotide-binding pocket regions (P-loop, switch 1) decreased, thereby blocking the opening motion between the two sub-domains of Gαs. (B) Change in mean squared fluctuation of Gαs following formation of Gαβγ(GDP) relative to Gαs in Gα(GTP). The change in mobility reflects the effects that GTP hydrolysis and Gβγ binding have on Gα.