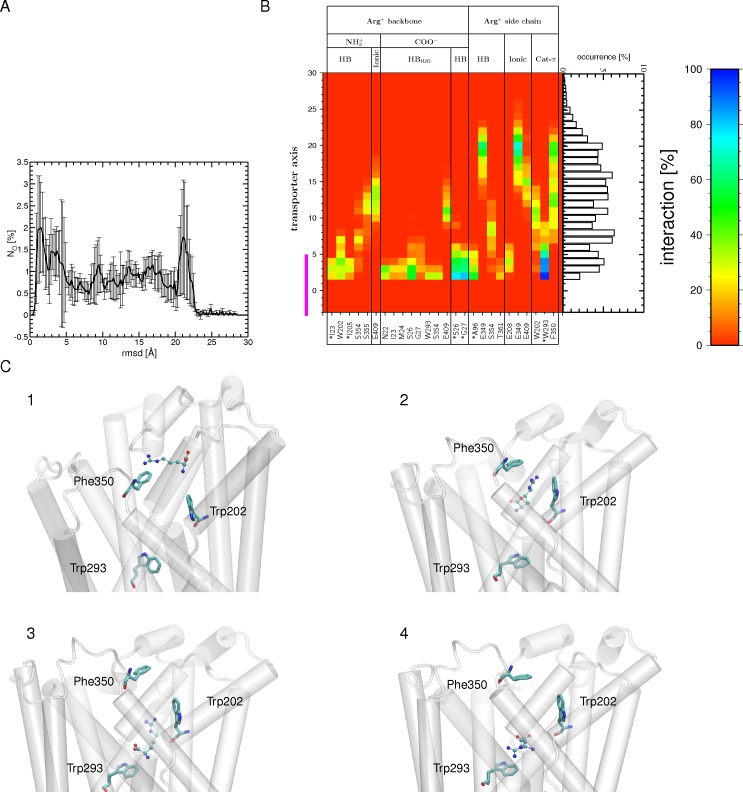
Fig 2. Binding of Arg+ to the OF open state during the tMDs.
(A) Number of occurrences (NO) for finding the substrate at a certain RSMD value, computed for the carbon atoms of Arg+ using all 6 tMD trajectories (each containing two AdiC monomers) and its crystal position in the OF open crystal structure (PDB ID 3OB6; Monomer A) as a reference. An RMSD value of zero Å corresponds to a perfect match between the MD conformation and the targeted crystal position of arginine. The standard error is shown as black bars. (B) Interactions (H bonds, ionic and cation-π interactions) formed between the Arg+ backbone or side chain and protein residues during its migration down to the binding site are shown, along with the occurrence of observing the center of mass of Arg+ at a certain position along the main axis of the transporter between the external medium and the binding site, as depicted by a bar graph representation. Only interactions with an occurrence higher than 20% in at least one bin width from all 12 binding events of the 6 tMD trajectories are shown. The abbreviations used for the different interactions are: ionic for ionic interaction, HB for H bond, HBH2O for water-mediated H bond, and Cat-π for cation π interaction. The binding site region (-5 to 5 Å) is highlighted by a magenta bar. (C) Four tMD conformations of AdiC highlighting Arg+ migration (1–4). The Arg+ side chain first forms a cation-π interaction with Phe350 (1). It then moves further down to form a sandwich configuration between the Phe350 and Trp202 side chains (2). Following this configuration, Arg+ slides down towards the binding site, forming a cation-π interaction with Trp202 (3). Arg+ leaves Trp202 to form a cation-π interaction with Trp293, which shapes the bottom of the binding site. Residues making persistent interactions with Arg+ are shown as sticks and the protein portion shaping the OF side is shown as a white cartoon. The Arg+ substrate is depicted as CPK.