FIGURE 7.
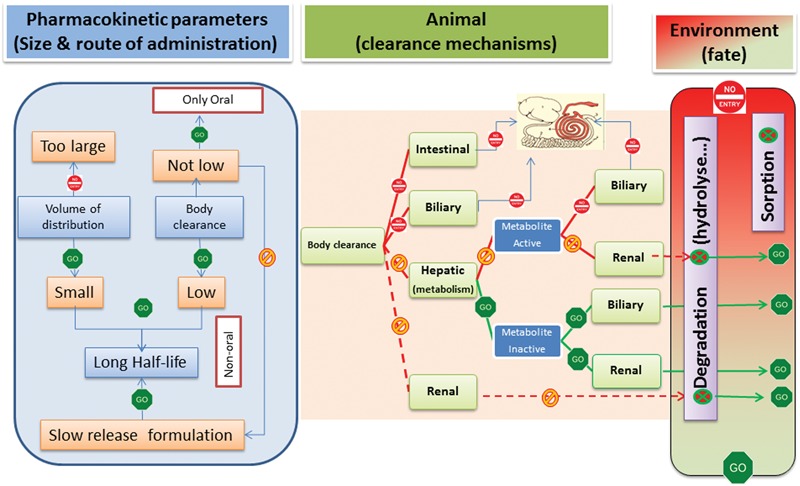
Pharmacokinetic profile of the ideal veterinary green AMD. The left panel indicates that the green AMD should not have a large volume of distribution. However, as volume of distribution is, with plasma clearance, one of two determinants of terminal half-life, an AMD with low volume of distribution will usually have too short a half-life. This is not a significant issue for the oral route, as an AMD with a short half-life can be administered virtually continually in feed or drinking water, in effect providing an oral infusion. In contrast, this is a major challenge for parenteral routes and development of slow release formulations (especially for AMDs exerting a time-dependent killing action) is necessary to ensure a long terminal half-life (flip-flop pharmacokinetics), thereby allowing both practicable and efficacious therapy with single dose administration. The middle panel indicates that a green AMD cannot have either intestinal or biliary clearance as a mechanism of elimination to ensure that there is no negative impact on the animal GIT microbiota. Clearance by hepatic metabolism is ideal, provided the metabolites are inactive. Alternatively, a high renal clearance is acceptable, provided the eliminated active drug is rapidly degraded in the environment or immobilized by physical sorption (right panel).
