Abstract
Studies proved that among all α1-adrenoceptors, cardiac myocytes functionally express only α1A- and α1B-subtype. Scientists indicated that α1A-subtype blockade might be beneficial in restoring normal heart rhythm. Therefore, we aimed to determine the role of α1-adrenoceptors subtypes (i.e., α1A and α1B) in antiarrhythmic effect of six structurally similar derivatives of 2-methoxyphenylpiperazine. We compared the activity of studied compounds with carvedilol, which is β1- and α1-adrenoceptors blocker with antioxidant properties. To evaluate the affinity for adrenergic receptors, we used radioligand methods. We investigated selectivity at α1-adrenoceptors subtypes using functional bioassays. We tested antiarrhythmic activity in adrenaline-induced (20 μg/kg i.v.), calcium chloride-induced (140 and 25 mg/kg i.v.) and barium chloride-induced (32 and 10 mg/kg i.v.) arrhythmia models in rats. We also evaluated the influence of studied compounds on blood pressure in rats, as well as lipid peroxidation. All studied compounds showed high affinity toward α1-adrenoceptors but no affinity for β1 receptors. Biofunctional studies revealed that the tested compounds blocked α1A-stronger than α1B-adrenoceptors, but except for HBK-19 they antagonized α1A-adrenoceptor weaker than α1D-subtype. HBK-19 showed the greatest difference in pA2 values—it blocked α1A-adrenoceptors around seven-fold stronger than α1B subtype. All compounds showed prophylactic antiarrhythmic properties in adrenaline-induced arrhythmia, but only the activity of HBK-16, HBK-17, HBK-18, and HBK-19 (ED50 = 0.18–0.21) was comparable to that of carvedilol (ED50 = 0.36). All compounds reduced mortality in adrenaline-induced arrhythmia. HBK-16, HBK-17, HBK-18, and HBK-19 showed therapeutic antiarrhythmic properties in adrenaline-induced arrhythmia. None of the compounds showed activity in calcium chloride- or barium chloride-induced arrhythmias. HBK-16, HBK-17, HBK-18, and HBK-19 decreased heart rhythm at ED84. All compounds significantly lowered blood pressure in normotensive rats. HBK-18 showed the strongest hypotensive properties (the lowest active dose: 0.01 mg/kg). HBK-19 was the only compound in the group, which did not show hypotensive effect at antiarrhythmic doses. HBK-16, HBK-17, HBK-18, HBK-19 showed weak antioxidant properties. Our results indicate that the studied 2-methoxyphenylpiperazine derivatives that possessed stronger α1A-adrenolytic properties (i.e., HBK-16, HBK-17, HBK-18, and HBK-19) were the most active compounds in adrenaline-induced arrhythmia. Thus, we suggest that the potent blockade of α1A-receptor subtype is essential to attenuate adrenaline-induced arrhythmia.
Keywords: arrhythmia, antiarrhythmic agents, α1-adrenolytics, 2-methoxyphenylpiperazine, α1A-adrenoceptor antagonist, α1B-adrenoceptor antagonist, α1D-adrenoceptor antagonist, hypotensive
Introduction
Arrhythmias are the most common causes of sudden cardiac death (Deo and Albert, 2012). Despite numerous antiarrhythmic drugs, pharmacotherapy is still ineffective in majority of patients. Moreover, all antiarrhythmic agents acting via different ion channels possess life-threatening proarrhythmic potential. Thus, scientists are still looking for effective and safe compounds, which will protect against arrhythmia and/or restore normal heart rhythm.
Antiarrhytmic activity of pharmacological agents resulting from their receptor-based mechanisms might be equally efficient and much safer than that observed for classical antiarrhythmic drugs. According to many studies, α1-adrenolytics may have potential in the treatment of arrhythmias. Scientists agree that the blockade of α1-, and particularly α1A-adrenoceptor may be beneficial in restoring normal heart rhythm (reviewed in Hieble, 2000 and Shannon and Chaudhry, 2006). The α1-adrenoceptor stimulation results in inositol trisphosphate (IP3) production, and subsequent Ca2+ release from the sarcoplasmatic reticulum (SR; Escobar et al., 2012). Although the regulation of Ca2+ level in cardiomyocytes mainly depends on ryanodine receptors and SR Ca2+ pump, the increased basal level of Ca2+ induced by IP3 may also alter the electrical excitability of cardiomyocytes, thus contributing to the development of arrhythmia e.g., atrial (Zima and Blatter, 2004) or ventricular fibrillation (Proven et al., 2006). Thereby, the blockade of α1-adrenoceptors may lead to the stabilization of Ca2+ level producing antiarrhythmic effect in arrhythmias induced by catecholamines e.g., catecholaminergic polymorphic ventricular tachycardia. The above hypothesis was supported by Suita et al. (2015), who demonstrated that prazosin not only shortened norepinephrine-induced elongation of atrial fibrillation in mice, but also attenuated norepinephrine-induced SR Ca2+ leak and spontaneous SR Ca2+ release in cultured atrium cardiomyocytes. This proves that α1-adrenoceptors may have role in preventing cardiac arrhythmias. Numerous animal studies confirmed this theory, showing antiarrhythmic properties of α1-adrenolytics (Sapa et al., 2011; Kubacka et al., 2013a; Rapacz et al., 2014, 2015b).
Since in our earlier experiments 2-methoxyphenylpiperazine derivatives showed high affinity toward α1-adrenoceptors (Pytka et al., 2015), in this study we aimed to determine the role of α1-adrenoceptors subtypes (i.e., α1A, α1B) in antiarrhythmic effect of six structurally similar derivatives of 2-methoxyphenylpiperazine. We compared the activity of studied compounds with carvedilol, which is β1- and α1-adrenoceptors blocker with antioxidant properties.
Materials and methods
Animals
The experiments were carried out using male normotensive Wistar rats [Krf: (WI) WU], weighing 200–250 g. Animals were kept in plastic cages (three rats per cage) at constant room temperature of 22 ± 2°C, with 12:12 h light/dark cycle. Rats had free access to food (standard pellet diet) and water. Each experimental and control groups consisted of four to six animals. The animals were killed by cervical dislocation immediately after the experiment. All injections were given in a volume of 1 ml/kg. All experimental procedures were approved by the Local Ethics Committee for Experiments on Animals of the Jagiellonian University in Krakow, Poland (approval numbers 110/2014 and 246/2015) and cared for in accordance with the Guide to the Care and Use of Experimental Animals.
Drugs
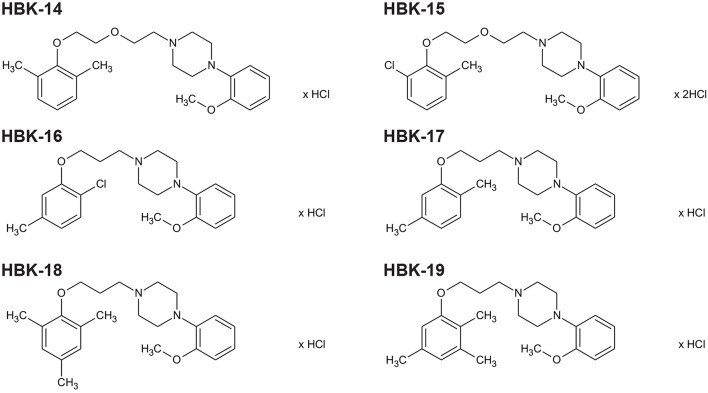
Six studied compounds (Figure 1): 1-[(2,6-dimethylphenoxy)ethoxyethyl]-4-(2-methoxyphenyl)piperazine hydrochloride (HBK14), 1-[(2-chloro-6-methylphenoxy)ethoxyethyl]-4-(2-methoxyphenyl)piperazine hydrochloride (HBK15), 1N-[3-(2-chloro-5-methylphenoxy)propyl]-4N-(2-methoxyphenyl)piperazine hydrochloride (HBK16), 1N-[3-(2,5-dimethylphenoxy)propyl]-4N-(2-methoxyphenyl)piperazine hydrochloride (HBK17), and 1N-[3-(2,4,6-trimethylphenoxy)propyl]-4N-(2-methoxyphenyl)piperazine hydrochloride (HBK18), 1N-[3-(2,3,5-trimethylphenoxy)propyl]-4N-(2-methoxyphenyl)piperazine hydrochloride (HBK-19) were synthesized in the Department of Bioorganic Chemistry, Chair of Organic Chemistry, Faculty of Pharmacy, Jagiellonian University (Waszkielewicz et al., 2015). The investigated compounds were dissolved in saline and administered intraperitoneally (i.p.) or intravenously (i.v.). Thiopental (Rotexmedica, Germany) was dissolved in saline and administered i.p. Chloroethylclonidine (Sigma, Germany), noradrenaline (Sigma, Germany), johimbine (Tocris, United Kingdom), propranolol (Fluka, USA), phentolamine (Sigma, Germany) were dissolved in saline or dimethyl sulfoxide (DMSO, Sigma, Germany) and used in radioligand or biofunctional studies. Adrenaline (Polfa S.A., Warsaw), carvedilol (Sigma, Germany), methoxamine (Sigma, China), calcium chloride (Fluka, Germany), and barium chloride (Sigma, Germany) were dissolved in saline and administered i.v. Heparin (Polfa S.A., Warsaw) was used as anticoagulant during experiments. The control groups received 0.9% NaCl solution. Other chemicals used were obtained from POCh (Polish Chemical Reagents, Poland).
Figure 1.
The chemical structures of tested 2-methoxyphenylpiperazine derivatives. HBK-14, 1-[(2,6-dimethylphenoxy)ethoxyethyl]-4-(2-methoxyphenyl)piperazine hydrochloride; HBK-15, 1-[(2-chloro-6-methylphenoxy)ethoxyethyl]-4-(2-meth oxyphenyl)piperazine hydrochloride; HBK-16, 1N-[3-(2-chloro-5-methylph enoxy)propyl]-4N-(2-methoxyphenyl)piperazine hydrochloride; HBK-17, 1N-[3-(2,5-dime thylphenoxy)propyl]-4N-(2-methoxyphenyl)piperazine hydrochloride; HBK-18, 1N-[3-(2,4,6-trimethylphenoxy)propyl]-4N-(2-methoxyphenyl)piperazine hydrochloride; HBK-19, 1N-[3-(2,3,5-trimethylphenoxy)propyl]-4N-(2-methoxyphenyl)piperazine hydrochloride.
Radioligand binding assay
The α1- and β1-adrenoceptor radioligand binding assay was performed on rat cerebral cortex. [3H]-prazosin (19.5 Ci/mmol, α1-adrenoceptor) and [3H]-CGP-12177 (48 Ci/mmol, β1-adrenergic receptor) were used. The brains were homogenized in 20 volumes of an ice-cold 50 mM Tris-HCl buffer (pH 7.6) and were centrifuged at 20,000 × g for 20 min (0−4°C). The cell pellet was resuspended in the Tris–HCl buffer and centrifuged again. Radioligand binding assays were performed in plates (MultiScreen/Millipore). The final incubation mixture (final volume 300 μl) consisted of 240 μl of the membrane suspension, 30 μl of [3H]-prazosin (0.2 nM), [3H]-CGP-12177 (0.2 nM) solution and 30 μl of the buffer containing seven to eight concentrations (10−11−10−4 M) of the tested compounds. In order to measure the unspecific binding, 10 μM phentolamine (for [3H]-prazosin) and 1 μM of propranolol (for [3H]-CGP-12177) were applied. The incubation was terminated by rapid filtration through glass fiber filters (Whatman GF/C) using a vacuum manifold (Millipore). The filters were then washed twice with the assay buffer and placed in scintillation vials with a liquid scintillation cocktail. Radioactivity was measured in a WALLAC 1409 DSA liquid scintillation counter (Perkin Elmer, USA). All the assays were made in duplicate and the inhibitory constants (Ki) were estimated.
Influence on α1A-adrenoceptors: rat tail artery
Rats were anesthetized with thiopental (75 mg/kg i.p.) and the middle part of the ventral caudal artery was removed, cleaned of surrounded tissues and uncovered of endothelium by gentle rubbing in the Krebs-Henseleit solution. The isolated artery, cut into ~4 mm long rings, was then horizontally put up between two stainless steel hooks (diameter 0.15 mm). One hook was fastened to the bottom of the chamber and the other to an isometric FDT10-A force displacement transducer (DMT Model 750TOBS, Denmark), linked with Powerlab 4/26 analyzer (ADInstruments), and processed by LabChart 7 software. The isolated rings were incubated in the Krebs-Henseleit solution (20 ml) at the temperature 37°C and pH 7.4 with constant oxygenation (O2/CO2, 19:1). The initial optimal tissues tension was set at 0.75 g. Chloroethylclonidine (3 μM) as the preferential α1B-adrenoreceptor alkylating agent was used during incubation and after 30 min it was completely washed off. Through 100 min of equilibration tissues were stimulated with noradrenaline (1 μM) four times with washing out until the contractile response become constant. Two cumulative concentration-response curves to noradrenaline, at an interval of 60 min, were established on each arterial ring both in the presence and absence of antagonist. The incubation with antagonists went on for 30 min. The experiments were carried out in the constant presence of yohimbine (0.1 μM) and propranolol (1 μM) to block α2- and β-adrenoceptors, respectively in order to minimize the involvement of these adrenoceptors in the response to noradrenaline.
Influence on α1B-adrenoceptors: mouse spleen
The influence on α1B-adrenoceptors was evaluated using the isolated mouse spleen. The spleen was removed from male mice right after killing the anesthetized animal by cervical dislocation. The isolated tissue was incubated in 20 ml cup filled with the Krebs-Henseleit solution at the temperature 37°C and pH 7.4 with constant oxygenation (O2/CO2, 19:1). The initial optimal tissues tension was set at 1.0 g. Through the 100 min of equilibration tissues were stimulated with noradrenaline (0.1−10.0 μM) three times with washing out until the contractile response become constant. Two cumulative concentration-response curves to noradrenaline, at an interval of 60 min, were established on each tissue both in the presence and absence of antagonist. The incubation with antagonists went on for 30 min. The experiments were carried out in the constant presence of propranolol (1 μM) to block β-adrenoceptors, and minimize the involvement of these adrenoceptors in the response to noradrenaline.
Influence on α1D-adrenoceptors: rat aorta
Finally, the influence on α1D-adrenoreceptors was investigated using the isolated rat aorta. Rats anesthetized with thiopental and killed by cervical dislocation had aorta removed, denuded of endothelium and incubated in the Krebs-Henseleit solution at the temperature 37°C and pH 7.4 with constant oxygenation (O2/CO2, 19:1). The aorta rings were maintained at optimal tension of 2.0 g. During 3 h of equilibration tissues were stimulated with noradrenaline three times (0.3 μM). Two cumulative concentration-response curves to noradrenaline, at an interval of 60 min, were established on each tissue both in the presence and absence of antagonist. The incubation with antagonists went on for 30 min. The experiments were carried out in the constant presence of yohimbine (0.1 μM) and propranolol (1 μM) to block α2- and β-adrenoceptors, respectively.
Prophylactic antiarrhythmic activity in adrenaline-, barium chloride-, and calcium chloride-induced arrhythmias
The procedures were performed according to the method described by Szekeres and Papp (1975). The heart rate disturbances were evoked by the intravenous administration of adrenaline (20 μg/kg), barium chloride (32 and 10 mg/kg) or calcium chloride (140 and 25 mg/kg) solution into the caudal vein in anesthetized rats (thiopental, 75 mg/kg). The studied compounds were administered i.p. 45 min before the injection of adrenaline, calcium chloride, or barium chloride. The electrocardiogram (ECG) was recorded during the first 2 min as well as in the 5th, 10th, and 15th min after the arrhythmogen injection. The criterion of antiarrhythmic activity was the lack of extrasystoles and inhibition of cardiac arrhythmia in comparison with the control group in adrenaline-induced arrhythmia or the progressive disappearance of the arrhythmia and reinstatement of the sinus rhythm in barium chloride- and calcium chloride-induced arrhythmias. The ED50 (a dose producing a 50% inhibition of ventricular contractions) with 95% confidence limits was determined by computer log-probit analysis according to Litchfield and Wilcoxon (1949) and Szekeres and Papp (1975). The compounds were administered at the dose 10 mg/kg. We gradually decreased the dose by half until the disappearance of antiarrhythmic activity.
Therapeutic antiarrhythmic activity in adrenaline-induced arrhythmia
The experiment was performed according to the method described by Szekeres and Papp (1968). The arrhythmia was evoked by the intravenous administration of adrenaline (20 μg/kg) into the caudal vein in anesthetized rats (thiopental, 75 mg/kg). The tested compounds were injected i.v. at the peak of arrhythmia directly after adrenaline injection, at the ED84 (a dose producing a 84% inhibition of premature ventricular contractions established in prophylactic adrenaline-induced arrhythmia). The range of doses was 0.325−0.504 mg/kg. The ECG was recorded constantly for 5 min as well as in the 10th and 15th min of the experiment. The criterion of antiarrhythmic activity was the reduction of premature ventricular contractions in comparison with the control group (Sapa et al., 2011).
The effect on normal electrocardiogram in rats
The experiment was carried out to exclude the influence of tested compounds on normal ECG. The ECG was recorded (ASPEL ASCARD B5 apparatus, standard lead II and paper speed of 50 mm/s) prior and also 5, 10, 15, 20, 30, 40, 50, and 60 min after the i.p. administration of tested compounds. The influence on QRS complex, PQ interval, heart rate (RR), and QTc interval was determined. The QTc was calculated according to the Bazzett's formula: QTc = QT/√RR (De Clerck et al., 2002). The compounds were administered at the ED84 (a dose producing a 84% inhibition of premature ventricular contractions established in prophylactic adrenaline-induced arrhythmia).
Influence on blood pressure in normotensive rats
Normotensive rats were anesthetized with thiopental (75 mg/kg ip). The right carotid artery was cannulated with a polyethylene tube filled with heparin solution to allow pressure measurements, using a Datamax apparatus (Columbus Instruments, USA; Kubacka et al., 2013b). The tested compounds were administered i.p. after 15 min of stabilization period. The compounds were administered at the dose 10 mg/kg. We gradually decreased the dose by half until the disappearance of antiarrhythmic activity.
Influence on blood vasopressor response in rats
To verify if the hypotensive activity was a result of α-adrenolytic properties, we studied the influence of tested compounds on the pressor response to methoxamine (150 μg/kg) The experiment was carried out for all active compounds, which were administered (i.v.) to the caudal vein at the lowest hypotensive dose (Kubacka et al., 2013b). Pressor response to methoxamine injected i.v. was measured before (control) and 5 min after the tested compounds. We administered the tested compounds at the lowest possible doses, not to lose selectivity for α1-adrenoceptors.
Antioxidant effect—lipid peroxidation in rat brain homogenate
This experiment was performed according to the method described by Yue et al. (1992). The rat brain homogenate containing 10 mg tissue/ml was prepared in 0.9% saline. The rates of membrane lipid peroxidation were measured by the formation of thiobarbituric acid reactive substances (TBARS). Rat brain homogenates (1 ml) were incubated at 37°C for 5 min with 10 μl of a tested compound or vehicle. Lipid peroxidation was initiated by the addition of 50 μl of 0.5 mM FeCl2 and 50 μl of 2.0 mM ascorbic acid. After 30 min of incubation, the reaction was stopped by adding 0.1 ml of 0.2% butylated hydroxytoluene (BHT). Thiobarbituric acid reagent was then added and the mixture was heated for 15 min in a boiling water bath. Carvedilol was used as reference compound. The compounds were tested at a concentration of 10−3 M. The TBARs were measured at 532 nm.
Data analysis
In radioligand binding studies, the obtained data were fitted to a one-site curve-fitting equation with Prism 6.0 (GraphPad Software), and inhibition constants (Ki) values were estimated from the Cheng−Prusoff equation (Cheng and Prusoff, 1973):
LO–labeled ligand concentration, KD–dissociation constant of labeled ligand
In functional bioassays the concentration–response curves were analyzed using GraphPad Prism 6.0 software (GraphPad Software Inc., San Diego, CA, USA) as previously described by Kubacka et al. (2013b). Data are means ± S.E.M. of at least 4 separate experiments. To establish Hill slopes for the agonist concentration–response curves and calculate EC50 values, curves were fitted to all the data by non-linear regression. The EC50 value in the presence and absence of antagonists was used to ascertain the concentration ratio (CR). Schild analysis was performed. If the slope was not significantly different from unity, the relative antagonistic potencies (pA2: −log of the concentration of an antagonist that doubles the concentration of the agonist needed to elicit the original submaximal response obtained in the absence of antagonist) were determined by plotting the log (CR-1) against the −log of antagonist concentration (Arunlakshana and Schild, 1959).
In case of in vivo experiments the results are presented as the means ± S.E.M. Statistically significant differences between groups were calculated using one-way analysis of variance (ANOVA) with repeated measurements followed by Dunnett's or Bonferroni's test post-hoc or Student's t-test. The criterion for significance was set at p < 0.05.
Antioxidant activity was expressed as the percentage reduction of the sample absorbance during the reaction at wavelength 532 nm.
The log-probit method described by Litchfield and Wilcoxon (1949) was used to determine median effective doses (ED50—doses producing 50% inhibition of premature ventricular contractions) and doses producing 84% of the maximal effect (ED84) for compounds in arrhythmia models.
Results
Affinity for adrenoceptors
All studied compounds possessed high affinity for α1-adrenoceptors but none of them bound to β1-adrenoceptors (Table 1).
Table 1.
The affinity of tested compounds for α1- and β1-adrenoceptors.
Functional affinity for α1A-, α1B-, and α1D-adrenoceptors
The tested compounds antagonized noradrenaline evoked contraction in isolated rat aorta and tail artery, as well as mouse spleen, and concentration-dependently, shifted the noradrenaline response to the right, without affecting the maximum response. The obtained pA2 values with Schild slopes not significantly different from unity indicated a competitive antagonism at α1A-, α1B-, and α1D-adrenoceptors (Tables 2A,B).
Table 2A.
The functional affinities of tested and reference compounds for α1-adrenergic receptor subtypes.
| Compound | Isolated tissues, α1-adrenoceptor subtypes, pA2 ± S.E.M. (slope ± S.E.M.) | ||
|---|---|---|---|
| Rat tail artery | Mouse spleen | Rat aorta | |
| α1A | α1B | α1D | |
| HBK-14 | 7.99 ± 0.09 (0.97 ± 0.15) | 7.70 ± 0.08 (1.03 ± 0.03) | 8.86 ± 0.06 (1.09 ± 0.10) |
| HBK-15 | 7.71 ± 0.07 (1.01 ± 0.18) | 7.67 ± 0.09 (0.96 ± 0.01) | 8.90 ± 0.09 (1.09 ± 0.09) |
| HBK-16 | 8.75 ± 0.03 (1.06 ± 0.08) | 8.21 ± 0.07 (1.03 ± 0.12) | 9.14 ± 0.03 (1.11 ± 0.12) |
| HBK-17 | 8.15 ± 0.08 (0.91 ± 0.05) | 7.94 ± 0.06 (0.91 ± 0.03) | 8.83 ± 0.09 (1.01 ± 0.07) |
| HBK-18 | 8.49 ± 0.09 (1.10 ± 0.06) | 8.35 ± 0.05 (1.03 ± 0.18) | 8.92 ± 0.08 (1.01 ± 0.05) |
| HBK-19 | 8.91 ± 0.09 (0.96 ± 0.05) | 8.07 ± 0.07 (1.06 ± 0.05) | 8.31 ± 0.08 (1.03 ± 0.09) |
| Prazosin | 8.93 ± 0.03a | 9.07 ± 0.09b | 8.85 ± 0.09b |
| Tamsulosin | 10.32 ± 0.05c | 8.33 ± 0.08d | 9.56 ± 0.07d |
The functional affinities were determined in the rat tail artery (α1A-adrenoceptor subtype), mouse spleen (α1B-adrenoceptor subtype), rat aorta (α1D-adrenoceptor subtype). Noradrenaline was used as α1-adrenergic receptor agonist. The highest pA2 values for each receptor subtype are in bold font. Antagonist potency of the tested compounds was expressed as pA2 ± S.E.M. pA2 was defined as −log of the concentration of an antagonist that doubles the concentration of the agonist necessary to elicit the original submaximal response in the absence of antagonist. pA2 values were obtained from the linear regression of Schild plot (Arunlakshana and Schild, 1959). Each value was the mean ± S.E.M. of 4–8 experimental results.
Parés-Hipólito et al. (2006).
Eltze (1996).
Jähnichen et al. (2004).
Eltze et al. (1999).
Table 2B.
The affinity of tested compounds for α1-adrenergic receptor subtypes.
| Compound | The affinity for α1-adrenergic receptor subtypes |
|---|---|
| HBK-14 | α1D > α1A > α1B |
| HBK-15 | α1D > α1A > α1B |
| HBK-16 | α1D > α1A > α1B |
| HBK-17 | α1D > α1A > α1B |
| HBK-18 | α1D > α1A > α1B |
| HBK-19 | α1A > α1D > α1B |
The functional affinities were determined in the rat tail artery (α1A-adrenoceptor subtype), mouse spleen (α1B-adrenoceptor subtype), rat aorta (α1D-adrenoceptor subtype).
HBK-14, HBK-15, HBK-16, HBK-17, and HBK-18 showed stronger antagonistic properties at α1D- than α1A- or α1B-adrenoceptors. HBK-19 antagonized α1A-adrenoceptors stronger than two other subtypes. All compounds antagonized α1A-adrenoceptors stronger than α1B-subtype. Among the studied compounds the strongest antagonist of α1A-adrenoceptor was HBK-19, α1B-adrenoceptor—HBK-18, and α1D-adrenoceptor—HBK-16.
The effect on normal electrocardiogram in rats
Table 3 shows the influence of tested compounds on normal ECG in rats.
Table 3.
The influence of the tested compounds on ECG.
| Compound | Parameters | Time of observation (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 30 | 40 | 50 | 60 | ||
| HBK-14 | Beats/min | 366.0 ± 10.8 | 369.8 ± 10.0 | 367.0 ± 9.9 | 359.8 ± 9.5 | 358.4 ± 11.4 | 359.8 ± 9.6 | 362.6 ± 11.9 | 361.6 ± 12.6 | 364.6 ± 11.2 |
| PQ (ms) | 47.1 ± 1.1 | 46.0 ± 1.0 | 46.3 ± 0.9 | 47.5 ± 1.3 | 46.3 ± 1.0 | 48.8 ± 1.9 | 48.2 ± 2.1 | 47.3 ± 1.6 | 48.4 ± 0.9 | |
| QRS (ms) | 26.1 ± 0.7 | 25.8 ± 0.5 | 27.6 ± 1.0 | 27.0 ± 0.6 | 26.3 ± 0.4 | 26.7 ± 0.9 | 26.1 ± 0.4 | 26.7 ± 1.0 | 27.5 ± 0.7 | |
| QT (ms) | 85.8 ± 1.3 | 85.0 ± 4.1 | 88.7 ± 1.9 | 89.0 ± 2.4 | 88.2 ± 2.9 | 89.6 ± 1.7 | 89.0 ± 2.1 | 87.9 ± 1.2 | 89.5 ± 2.4 | |
| QTc (ms) | 211.7 ± 3.2 | 209.2 ± 8.3 | 214.8 ± 5.0 | 212.9 ± 5.7 | 212.5 ± 4.6 | 214.1 ± 5.0 | 214.1 ± 5.2 | 210.9 ± 5.1 | 214.1 ± 4.2 | |
| HBK-15 | Beats/min | 353.8 ± 10.2 | 365.4 ± 15.3 | 350.8 ± 10.5 | 333.9 ± 12.4 | 327.6 ± 20.3 | 340.1 ± 17.7 | 339.5 ± 20.4 | 350.1 ± 17.9 | 351.8 ± 15.0 |
| PQ (ms) | 48.7 ± 1.0 | 48.4 ± 0.5 | 48.2 ± 1.0 | 46.6 ± 0.9 | 47.6 ± 0.5 | 46.1 ± 0.3 | 46.1 ± 0.7 | 47.5 ± 0.7 | 48.0 ± 1.1 | |
| QRS (ms) | 25.7 ± 0.7 | 26.3 ± 0.9 | 26.1 ± 1.1 | 25.6 ± 1.2 | 26.3 ± 0.5 | 26.4 ± 0.9 | 28.0 ± 0.9 | 26.1 ± 1.2 | 26.3 ± 0.5 | |
| QT (ms) | 87.3 ± 1.4 | 86.5 ± 1.3 | 87.2 ± 2.0 | 87.5 ± 2.0 | 87.2 ± 0.6 | 86.7 ± 1.1 | 87.0 ± 1.5 | 88.2 ± 0.8 | 88.5 ± 2.2 | |
| QTc (ms) | 212.0 ± 5.0 | 213.6 ± 7.0 | 211.3 ± 6.2 | 207.1 ± 6.8 | 203.7 ± 7.2 | 206.4 ± 7.9 | 206.5 ± 6.6 | 212.7 ± 6.9 | 213.9 ± 7.2 | |
| HBK-16 | Beats/min | 329.6 ± 9.8 | 331.4 ± 12.5 | 330.1 ± 12.4 | 319.4 ± 10.3 | 310.6 ± 9.8 | 302.8 ± 11.0 | 294.1 ± 12.2a | 291.4 ± 13.0b | 288.0 ± 13.8b |
| PQ (ms) | 64.4 ± 1.1 | 63.7 ± 1.1 | 63.6 ± 0.9 | 63.9 ± 1.1 | 65.1 ± 1.2 | 64.4 ± 1.1 | 65.9 ± 0.8 | 67.3 ± 1.6 | 68.2 ± 0.9 | |
| QRS (ms) | 21.7 ± 2.2 | 21.7 ± 2.2 | 21.7 ± 2.2 | 21.7 ± 2.2 | 21.7 ± 2.2 | 21.7 ± 2.2 | 21.7 ± 2.2 | 22.0 ± 2.1 | 22.3 ± 2.0 | |
| QT (ms) | 51.4 ± 3.5 | 51.2 ± 3.5 | 51.2 ± 3.3 | 50.1 ± 3.6 | 51.4 ± 3.1 | 52.7 ± 3.7 | 53.5 ± 2.8 | 54.7 ± 2.8 | 55.5 ± 2.4 | |
| QTc (ms) | 120.5 ± 8.7 | 120.7 ± 9.7 | 120.2 ± 8.8 | 115.8 ± 9.6 | 117.3 ± 8.7 | 119.0 ± 10.3 | 118.9 ± 8.4 | 121.1 ± 8.7 | 122.0 ± 7.7 | |
| HBK-17 | Beats/min | 365.2 ± 15.0 | 359.4 ± 16.6 | 348.7 ± 15.0 | 341.1 ± 13.4 | 333.5 ± 13.3b | 327.4 ± 11.8c | 333.0 ± 10.6b | 323.8 ± 14.1c | 320.9 ± 13.4c |
| PQ (ms) | 66.5 ± 3.7 | 65.0 ± 3.3 | 64.3 ± 3.4 | 65.6 ± 3.7 | 65.9 ± 3.4 | 67.7 ± 4.0 | 67.0 ± 3.2 | 67.4 ± 3.5 | 67.6 ± 3.3 | |
| QRS (ms) | 23.3 ± 1.8 | 23.3 ± 1.4 | 23.0 ± 1.7 | 23.3 ± 2.0 | 22.7 ± 1.8 | 22.7 ± 2.0 | 23.0 ± 1.7 | 23.3 ± 1.4 | 23.0 ± 1.7 | |
| QT (ms) | 55.2 ± 2.3 | 55.3 ± 0.9 | 55.7 ± 1.1 | 57.4 ± 0.8 | 57.7 ± 1.8 | 60.3 ± 1.1 | 61.3 ± 1.6 | 60.4 ± 2.0 | 61.5 ± 2.3 | |
| QTc (ms) | 135.8 ± 5.3 | 135.2 ± 3.3 | 134.1 ± 3.1 | 136.5 ± 2.3 | 135.6 ± 3.5 | 140.7 ± 3.7 | 144.3 ± 5.0 | 140.2 ± 5.4 | 142.0 ± 5.0 | |
| HBK-18 | Beats/min | 328.0 ± 7.4 | 306.9 ± 9.0 | 298.7 ± 8.9 | 284.7 ± 7.6a | 273.9 ± 8.0b | 267.4 ± 17.5c | 260.3 ± 22.8c | 258.9 ± 25.4c | 252.3 ± 25.1c |
| PQ (ms) | 61.3 ± 4.6 | 61.5 ± 6.3 | 57.4 ± 4.2 | 55.8 ± 1.6 | 51.9 ± 3.8 | 56.9 ± 3.5 | 57.4 ± 3.1 | 59.0 ± 3.8 | 59.6 ± 4.7 | |
| QRS (ms) | 28.1 ± 3.1 | 30.4 ± 3.2 | 30.8 ± 3.6 | 27.0 ± 2.9 | 29.1 ± 2.7 | 28.5 ± 2.8 | 28.6 ± 2.8 | 27.6 ± 3.0 | 30.9 ± 3.8 | |
| QT (ms) | 73.9 ± 6.2 | 77.0 ± 7.0 | 77.7 ± 4.9 | 76.1 ± 5.8 | 77.9 ± 4.2 | 75.3 ± 3.2 | 76.9 ± 4.9 | 76.0 ± 2.9 | 76.7 ± 3.5 | |
| QTc (ms) | 172.4 ± 13.5 | 174.2 ± 16.3 | 173.4 ± 11.7 | 166.4 ± 14.5 | 166.9 ± 11.1 | 159.0 ± 9.7 | 160.4 ± 14.4 | 157.2 ± 9.9 | 157.1 ± 12.0 | |
| HBK-19 | Beats/min | 355.3 ± 19.7 | 349.7 ± 25.3 | 339.1 ± 21.0 | 280.1 ± 1.1c | 277.2 ± 5.0c | 278.2 ± 6.7c | 332.8 ± 18.9 | 396.8 ± 31.8 | 398.0 ± 32.4 |
| PQ (ms) | 56.7 ± 0.7 | 56.6 ± 0.0 | 56.0 ± 0.0 | 58.2 ± 1.8 | 60.1 ± 1.3 | 60.1 ± 1.7 | 59.3 ± 0.7 | 55.1 ± 0.3 | 56.3 ± 1.1 | |
| QRS (ms) | 35.7 ± 1.0 | 34.7 ± 1.6 | 34.0 ± 0.3 | 32.0 ± 1.2 | 32.7 ± 0.4 | 34.3 ± 0.2 | 32.0 ± 3.1 | 36.0 ± 1.5 | 30.3 ± 1.1 | |
| QT (ms) | 76.4 ± 8.2 | 76.9 ± 1.1 | 77.5 ± 0.5 | 82.2 ± 2.2 | 84.3 ± 1.1 | 82.2 ± 0.4 | 81.5 ± 2.5 | 76.5 ± 0.5 | 80.2 ± 0.4 | |
| QTc (ms) | 186.9 ± 24.6 | 185.5 ± 9.5 | 184.2 ± 6.9 | 177.6 ± 4.4 | 181.1 ± 4.1 | 177.0 ± 3.0 | 192.2 ± 11.2 | 196.2 ± 6.9 | 206.3 ± 9.6 | |
Rats were anesthetized intraperitoneally (i.p.) with thiopental (75 mg/kg). The compounds were administered (i.p.) at the ED84 obtained in prophylactic adrenaline-induced arrhythmia i.e., 6.154 mg/kg (HBK-14), 20.218 mg/kg (HBK-15), 0.363 mg/kg (HBK-16), 0.504 mg/kg (HBK-17), 0.325 mg/kg (HBK-18), 0.444 mg/kg (HBK-19). The data are expressed as mean ± S.E.M. Statistical analysis: one-way ANOVA with repeated measurements, Dunnet post-hoc test,
p < 0.05,
p < 0.01,
p < 0.001 vs. initial values. n = 4–6 rats per group.
HBK-14 and HBK-15 administered at the dose 6.154 and 20.218 mg/kg, respectively did not influence the ECG parameters throughout the experiment. HBK-16 at the dose 0.363 mg/kg did not influence PQ interval, QRS complex or QTc interval but it significantly reduced heart rate by 11−13%, since the 40th min of the observation. Similarly, HBK-17 at the dose 0.504 mg/kg significantly decreased heart rate by 9−12%, 20 min after administration, without affecting PQ interval, QRS complex or QTc interval. HBK-18 at the dose 0.325 mg/kg significantly reduced the heart rate by 13−23%, since the 15th min of the observation and did not affect PQ interval, QRS complex or QTc interval. HBK-19 at the dose 0.444 mg/kg between the 15th and 30th min after administration reduced heart rate by 21−22% without the influence on PQ interval, QRS complex, or QTc interval.
Prophylactic antiarrhythmic activity in adrenaline-, barium chloride-, and calcium chloride-induced arrhythmias
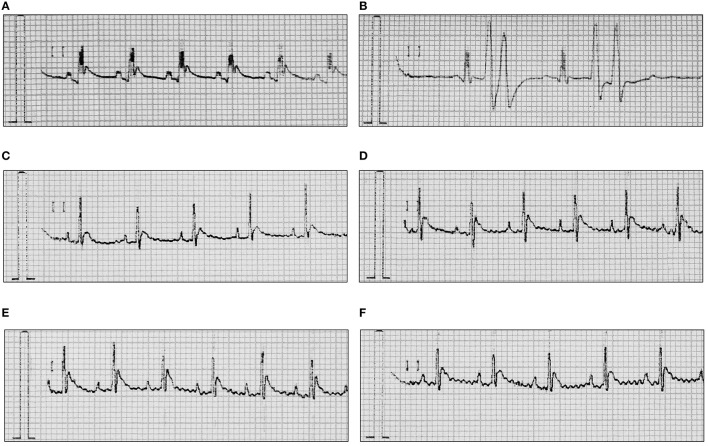
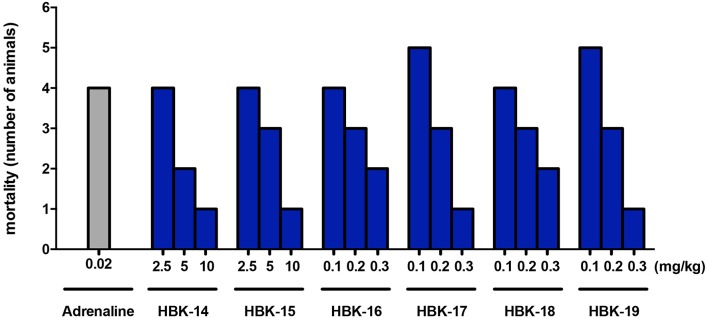
In anesthetized rats i.v. injection of adrenaline (20 μg/kg) caused atrioventricular disturbances, ventricular and supraventricular extrasystoles in 100% of the animals, which led to the death of ~70% of the animals. The studied compounds administered 45 min (i.p.) prior to adrenaline, decreased the number of extrasystoles and mortality (Figures 2, 3, Table 4). Table 5 shows the ED50 values (doses producing 50% inhibition of premature ventricular contractions).
Figure 2.
Representative ECGs after treatment with adrenaline and/or HBK-16, HBK-17, HBK-18, and HBK-19 in rats. (A) Normal reading (Control). (B) Arrhythmia control—adrenaline (20 μg/kg, i.v.). (C) Adrenaline-induced arrhythmia (20 μg/kg, i.v.)+HBK-16 (0.3 mg/kg, i.v. injection 45 min prior to adrenaline). (D) Adrenaline-induced arrhythmia (20 μg/kg, i.v.)+HBK-17 (0.3 mg/kg, i.v. injection 45 min prior to adrenaline). (E) Adrenaline-induced arrhythmia (20 μg/kg, i.v.)+HBK-18 (0.3 mg/kg, i.v. injection 45 min prior to adrenaline). (F) Adrenaline-induced arrhythmia (20 μg/kg, i.v.)+HBK-19 (0.3 mg/kg, i.v. injection 45 min prior to adrenaline).
Figure 3.
The effect of adrenaline and studied compounds on mortality of rats in prophylactic adrenaline-induced arrhythmia. Rats were anesthetized with intraperitoneal (i.p.) injection of thiopental (75 mg/kg). The tested compounds were administered (i.p.) 45 min before the experiment. The observation was carried out for 15 min after the intravenous injection of adrenaline (20 μg/kg). n = 5−6 animals per group.
Table 4.
The antiarrhythmic activity of 2-methoxy phenylpiperazine derivatives in prophylactic model of adrenaline induced arrhythmia.
| Compound | Dose (mg/kg) | Extrasystoles (%) | Bigeminy (%) | Bradycardia (%) | Blocks (%) |
|---|---|---|---|---|---|
| Adrenaline | – | 66.7 | 33.3 | 66.7 | 50.0 |
| HBK-14 | 2.5 | 100.0 | 50.0 | 83.3 | 83.3 |
| 5 | 50.0 | 33.3 | 50.0 | 33.3 | |
| 10 | 16.7 | 16.7 | 50.0 | 33.3 | |
| HBK-15 | 2.5 | 66.7 | 33.3 | 66.7 | 66.7 |
| 5 | 50.0 | 16.7 | 66.7 | 50.0 | |
| 10 | 33.3 | 16.7 | 50.0 | 33.3 | |
| HBK-16 | 0.1 | 83.3 | 33.3 | 66.7 | 50.0 |
| 0.2 | 33.3 | 16.7 | 50.0 | 33.3 | |
| 0.3 | 16.7 | – | 33.3 | 16.7 | |
| HBK-17 | 0.1 | 66.7 | 50.0 | 67.7 | 67.7 |
| 0.2 | 50.0 | 33.3 | 50.0 | 33.3 | |
| 0.3 | 16.7 | 16.7 | 33.3 | 16.7 | |
| HBK-18 | 0.1 | 83.3 | 50.0 | 66.7 | 66.7 |
| 0.2 | 50.0 | 16.7 | 50.0 | 33.0 | |
| 0.3 | 16.7 | 12.0 | 16.3 | 16.3 | |
| HBK-19 | 0.1 | 66.7 | 33.3 | 66.7 | 50.0 |
| 0.2 | 50.0 | 16.7 | 33.3 | 33.3 | |
| 0.3 | 16.7 | – | 16.7 | 16.7 |
Rats were anesthetized with intraperitoneal (i.p.) injection of thiopental (75 mg/kg). The tested compounds were administered (i.p.) 45 min before the experiment. The observation was carried out for 15 min after the intravenous injection of adrenaline (20 μg/kg) i.e., during the first 2 min, in the 5, 10, and 15th min. n = 5−6 animals per group.
Table 5.
Prophylactic antiarrhythmic activities of tested compounds and carvedilol in adrenaline-induced arrhythmia.
| Compound | ED50(mg/kg) |
|---|---|
| HBK-14 | 3.88 (2.68−5.61) |
| HBK-15 | 4.80 (2.32−9.93) |
| HBK-16 | 0.20 (0.10−0.39) |
| HBK-17 | 0.20 (0.12−0.35) |
| HBK-18 | 0.18 (0.11−0.29) |
| HBK-19 | 0.21 (0.13−0.34) |
| Carvedilol | 0.36 (0.16−0.80) |
Rats were anesthetized with intraperitoneal (i.p.) injection of thiopental (75 mg/kg). The tested compounds were administered (i.p.) 45 min before the experiment. The observation was carried out for 15 min after the intravenous injection of adrenaline (20 μg/kg) i.e., during the first 2 min, in the 5, 10, and 15th min. The ED50 values with confidence limits were calculated according to the methods described by Litchfield and Wilcoxon (1949). Each value was obtained from three experimental groups. n = 5−6 animals per group.
The injection (i.v.) of barium chloride (32 mg/kg) caused rapid ventricular extrasystoles in all animals (100%), which led to the death within 3−5 min. Lower dose of barium chloride (10 mg/kg) caused ventricular extrasystoles in around 60% of rats and did not lead to the death of animals. We did not observe a reproducible negative effect on heart rhythm when barium chloride was used at the dose 10 mg/kg. None of the tested compounds administered (i.p.) 45 min before barium chloride were active in barium chloride-induced model of arrhythmia (data not shown).
In anesthetized rats injection (i.v.) of calcium chloride (140 mg/kg) caused rapid ventricular fibrillation in all animals (100%), which led to death within 3−5 min. The intravenous administration of calcium chloride (25 mg/kg) caused ventricular fibrillation in all animals, but did not lead to the death of rats. None of the tested compounds administered (i.p.) 45 min prior to calcium chloride were active in the above model of arrhythmia (data not shown).
Therapeutic antiarrhythmic activity in adrenaline-induced arrhythmia
All tested compounds administered i.v. at the peak of adrenaline-induced arrhythmia (20 μg/kg) reduced the number of premature ventricular contractions (Figure 4).
Figure 4.
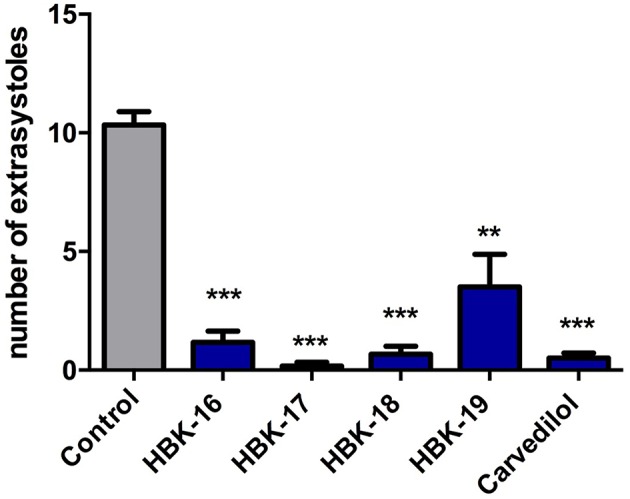
Therapeutic antiarrhythmic activities of tested compounds in adrenaline-induced arrhythmia. Rats were anesthetized with intraperitoneal injection of thiopental (75 mg/kg). The tested compounds were administered intravenously (i.v.) at the ED84 obtained in prophylactic adrenaline-induced arrhythmia i.e., 0.363 mg/kg (HBK-16), 0.504 mg/kg (HBK-17), 0.325 mg/kg (HBK-18), 0.444 mg/kg (HBK-19), 0.979 mg/kg (carvedilol). Data are reported as means ± S.E.M. Statistical analysis: Student's t-test; **p < 0.01, ***p < 0.001. n = 5−6 animals per group.
Influence on blood pressure in normotensive rats
Table 6 presents the lowest dose of each tested compound, which significantly lowered systolic and diastolic blood pressure in rats.
Table 6.
The hypotensive activity of 2-methoxyphenylpiperazine derivatives in normotensive rats.
| Compound | Dose (mg/kg) | Pressure | Time of observation (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 5 | 10 | 20 | 30 | 40 | 50 | 60 | |||
| HBK-14 | 0.625 | Systolic | 138.3 ± 6.8 | 135.2 ± 7.3 | 131.2 ± 6.8c | 127.3 ± 6.6c | 125.3 ± 7.0c | 124.2 ± 7.0c | 127.3 ± 6.3c | 128.2 ± 5.4c |
| Diastolic | 106.7 ± 4.3 | 105.2 ± 6.1 | 103.0 ± 5.8 | 97.3 ± 6.0c | 95.2 ± 6.1c | 92.3 ± 6.2c | 94.0 ± 5.8c | 98.0 ± 5.1c | ||
| HBK-15 | 5.0 | Systolic | 139.0 ± 2.7 | 121.2 ± 4.8c | 116.3 ± 6.0c | 112.0 ± 5.5c | 112.2 ± 4.2c | 112.5 ± 4.4c | 113.2 ± 3.7c | 117.3 ± 3.0c |
| Diastolic | 110.3 ± 2.2 | 96.3 ± 3.7c | 92.0 ± 4.3c | 88.5 ± 3.5c | 88.3 ± 2.9c | 91.0 ± 3.2c | 90.2 ± 2.7c | 93.0 ± 1.2c | ||
| HBK-16 | 0.1 | Systolic | 124.7 ± 3.1 | 123.2 ± 3.3 | 121.2 ± 3.2 | 114.8 ± 2.0b | 113.0 ± 2.4c | 110.3 ± 2.1c | 110.8 ± 2.0b | 110.7 ± 1.9c |
| Diastolic | 97.0 ± 4.3 | 95.0 ± 5.1 | 93.7 ± 5.1 | 91.3 ± 5.0b | 89.8 ± 5.2a | 87.7 ± 4.7b | 88.0 ± 4.3c | 87.7 ± 4.6b | ||
| HBK-17 | 0.1 | Systolic | 136.8 ± 3.7 | 132.7 ± 4.8 | 130.5 ± 4.7 | 125.5 ± 4.9b | 124.2 ± 4.4b | 121.7 ± 3.9b | 121.5 ± 3.4a | 121.2 ± 3.9b |
| Diastolic | 110.7 ± 4.6 | 108.2 ± 5.2 | 105.8 ± 4.3 | 99.3 ± 4.7a | 96.8 ± 4.0b | 94.0 ± 3.7b | 93.3 ± 4.0b | 94.5 ± 4.3b | ||
| HBK-18 | 0.01 | Systolic | 125.7 ± 8.2 | 117.7 ± 9.1 | 112.7 ± 7.7 | 96.5 ± 8.9b | 93.8 ± 8.7c | 91.8 ± 10.0b | 90.7 ± 8.6b | 90.2 ± 9.2a |
| Diastolic | 94.3 ± 6.4 | 90.7 ± 7.1 | 85.5 ± 7.5 | 79.5 ± 7.8a | 78.3 ± 7.2a | 78.8 ± 7.0b | 78.7 ± 5.6c | 77.5 ± 6.0b | ||
| HBK-19 | 0.625 | Systolic | 127.0 ± 4.0 | 121.0 ± 4.0b | 116.0 ± 3.5c | 111.5 ± 3.2c | 110.0 ± 2.3c | 107.5 ± 3.2c | 106.0 ± 3.5c | 106.0 ± 4.0c |
| Diastolic | 98.0 ± 4.6 | 94.5 ± 2.6 | 87.5 ± 0.4c | 89.0 ± 2.3b | 87.0 ± 2.9c | 84.5 ± 1.4c | 83.5 ± 1.4c | 84.0 ± 0.6c | ||
Rats were anesthetized intraperitoneally (i.p.) with thiopental (75 mg/kg). The compounds were administered (i.p.). The results present the lowest hypotensive dose. The data are the means of six experiments ± S.E.M. Statistical analysis: one-way ANOVA test with repeated measurements, Dunnet post-hoc test.
p < 0.05,
p < 0.01,
p < 0.001 vs. control values. n = 6 rats per group.
HBK-14 at the dose 0.625 mg/kg, 10 min after injection, significantly reduced systolic blood pressure by 5−10%, and diastolic blood pressure by 9−14% since the 20th min of the observation. HBK-15 at the dose 5.0 mg/kg significantly reduced systolic blood pressure by 13−19%, and diastolic blood pressure by 13−20%, 5 min after administration. HBK-16 at the dose 0.1 mg/kg, 20 min after administration, significantly reduced systolic blood pressure by 8−11% and diastolic blood pressure by 6−10%. HBK-17 at the dose 0.1 mg/kg significantly reduced systolic blood pressure by 8−11% and diastolic blood pressure by 10−16%, 20 min after administration. HBK-18 at the dose 0.01 mg/kg, from the 20th min of the observation, significantly reduced systolic and diastolic blood pressure by 23−28 and 16−18%, respectively. HBK-19 at the dose 0.625 mg/kg since the 5th min after i.p. administration, significantly reduced systolic blood pressure by 5−17%, whereas diastolic blood pressure by 9−15% since the 10th min of the observation.
Influence on blood vasopressor response in rats
In the control group the increase of blood pressure after methoxamine (150 μg/kg) was ranging from 62.7 ± 10.4 to 94.2 ± 2.7 mmHg. Figure 5 shows that all tested compounds at the lowest hypotensive doses, significantly antagonized the pressor response to methoxamine.
Figure 5.
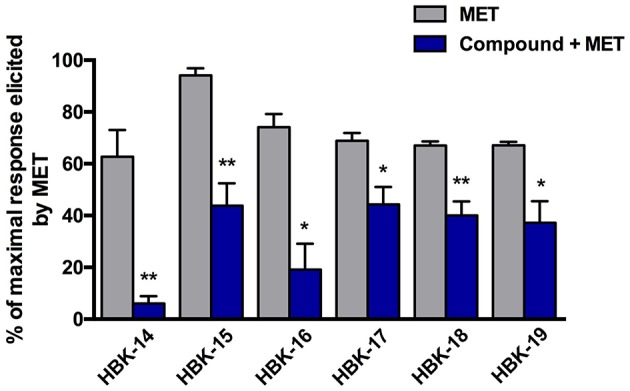
The effect of tested compounds on the blood pressure response to methoxamine. Rats were anesthetized with intraperitoneal injection of thiopental (75 mg/kg). The studied compounds were administered intravenously (i.v.), at the lowest hypotensive doses i.e., 0.625 mg/kg (HBK-14), 5.0 mg/kg (HBK-15), 0.1 mg/kg (HBK-16), 0.1 mg/kg (HBK-17), 0.01 mg/kg (HBK-18), 0.625 mg/kg (HBK-19). Methoxamine (MET) was administered at the dose 150 μg/kg (i.v.). All values represent means ± S.E.M. Statistical analysis: Student's t-test; *p < 0.05, **p < 0.01, compared to the initial maximal response (obtained before the administration of tested compounds). n = 5−6 animals per group.
Influence on lipid peroxidation in rat brain homogenate
Carvedilol, HBK-16, HBK-17, HBK-18, and HBK-19 inhibited lipid peroxidation (Table 7). HBK-14 and HBK-15 were inactive in this test.
Table 7.
The influence of the tested compounds on lipid peroxidation in rat brain homogenate—antioxidant effect.
| Compound | Absorbance | % reduction of absorbance (% antioxidant activity) |
|---|---|---|
| HBK-14 | 0.939 ± 0.007 | −4.10 |
| HBK-15 | 0.891 ± 0.008 | 1.22 |
| HBK-16 | 0.752 ± 0.007 | 16.63 |
| HBK-17 | 0.773 ± 0.009 | 14.30 |
| HBK-18 | 0.781 ± 0.004 | 13.41 |
| HBK-19 | 0.698 ± 0.007 | 22.62 |
| Carvedilol | 0.094 ± 0.002 | 89.58 |
The rates of membrane lipid peroxidation were measured using rat brain homogenate by the formation of thiobarbituric acid reactive substances (TBARs). The studied compounds were tested at a concentration of 10−3 M. The TBARs were measured at 532 nm.
Discussion
We found that the studied 2-methoxyphenylpiperazine derivatives that possessed stronger α1A-adrenolytic properties (i.e., HBK-16, HBK-17, HBK-18, and HBK-19) were the most active compounds in adrenaline-induced arrhythmia. Their antiarrhythmic (but not antioxidant) activity was comparable to that of carvedilol. The tested compounds showed hypotensive effect resulting from their α1-adrenolytic properties. HBK-19 was the only compound in the group that did not lower blood pressure at antiarrhythmic doses.
Scientists reported that 2-methoxyphenylpiperazine derivatives often interact with adrenergic receptors (Handzlik et al., 2008; Kubacka et al., 2013a; Rapacz et al., 2014, 2015a,b). Thus, we evaluated the affinity of the studied compounds for α1-and β1-adrenoceptors. The radioligand studies revealed that HBK-16, HBK-17, HBK-18, and HBK-19 possessed high affinity for α1-, but not β1-adrenoceptors. This is in agreement with our previous experiments on 2-methoxyphenylpiperazine derivatives i.e., HBK-14 and HBK-15, which showed no affinity for β1- and high affinity for α1-adrenoreceptors (Pytka et al., 2015).
Studies proved that cardiac myocytes functionally express α1A- and α1B-adrenoceptors. O'Connell et al. (2003) demonstrated that despite the presence of α1D-adrenoceptors mRNA, rodent cardiac myocytes did not express α1D-subtype protein by binding. However, Chalothorn et al. (2003) showed that α1D-adrenoceptors might be expressed in the coronary vasculature. Thus, α1A- and α1B-adrenoceptors may play important role in the development of arrhythmias induced by catecholamines, while α1D-subtype in ischemia-induced arrhythmias.
Scientists indicated that agents which block α1A-adrenoceptors may have antiarrhythmic potential (Hieble, 2000). Harrison et al. (1998) showed that hearts from transgenic rats expressing constitutively active α1B-adrenoceptors, and having 50% reduced α1A-mRNA levels, were less sensitive to ischemia-induced ventricular tachycardia than normal rats. Lee and Rosen (1993) proved that the blockade of α1B receptors by chlorethylclonidine increased the amplitude of delayed afterdepolarizations induced by calcium and phenylephrine. Altogether, these findings suggest that agents, which block α1A-adrenoceptors stronger than α1B subtype, may have antiarrhythmic potential.
Therefore, we determined the selectivity of studied compounds at α1-adrenoceptor subtypes. Biofunctional assays revealed that all compounds competitively blocked α1A, α1B, and α1D subtypes. HBK-19 and HBK-16 were the strongest α1A-adrenoceptor antagonists, while HBK-14 and HBK-15 were the weakest. Although we did not find highly selective compounds, all 2-methoxyphenylpiperazine derivatives antagonized α1A-adrenoceptors stronger than α1B subtype. HBK-19 showed the greatest difference in pA2 values—it blocked α1A-adrenoceptors around seven-fold stronger than α1B subtype.
Since all studied compounds blocked α1A- and α1B-adrenoceptors, we decided to examine their antiarrhythmic activity. We also investigated whether antiarrhythmic activity depended on the strength of α1A-adrenoceptor blockade or the differences between pA2 values for α1A- and α1B-adrenoceptors. To determine antiarrhythmic effect, we used three models of arrhythmia i.e., adrenaline-, barium chloride-, and calcium chloride-induced.
All compounds showed antiarrhythmic activity in adrenaline-induced model of arrhythmia, and reduced mortality of rats. HBK-18 possessed the strongest prophylactic antiarrhythmic properties, but ED50 values for HBK-16, HBK-17, and HBK-19 were also very low. Except for HBK-14 and HBK-15, prophylactic antiarrhythmic activities of compounds in adrenaline-induced arrhythmia were comparable to that of carvedilol (reference drug). We think that the weak antiarrhythmic activity of HBK 14 and HBK 15 may be due to their weaker α1-adrenolytic properties (see Table 2), which are crucial for antiarrhythmic effect in the applied model of arrhythmia. Since the compounds used in the experiments did not present potent selectivity toward different subtypes of α1−adrenoceptors, we cannot definitely conclude which receptor subtype should be primarily blocked to achieve antiarrhythmic effect. Although, HBK-19 showed the greatest difference in pA2 values for the α1A- and α1B receptor subtypes, it did not possess the strongest antiarrhythmic properties. Similarly, according to the studies performed by Koshimizu et al. (2004), the pA2 value for α1A−adrenoceptor subtype for carvedilol was 9.0, whereas for α1B−adrenoceptor was 10.0. Carvedilol showed comparable properties in adrenaline-induced arrhythmia model. Thus, we claim that the potent blockade of α1A-receptor subtype is essential to attenuate adrenaline-induced arrhythmia, but the role of α1B-adrenoceptor blockade needs further studies. Carvedilol is a potent β1- and α1-adrenoceptors blocker with antioxidant activity. Surprisingly, despite the fact that carvedilol blocked both β1- and α1-adrenoceptors, and the studied compounds only α1-adrenoceptors, their antiarrhythmic effect was comparable. Therefore, we suggest that this may indicate more important role of α1- than β1-adrenoceptors blockade in adrenaline-induced arrhythmia model.
Arrhythmia models induced by calcium or sodium chloride are associated with the changes in intracellular ion concentration. These changes are ion channel dependent, and their dynamics and amplitude are high. In arrhythmias induced by adrenaline, the stimulation of adrenergic receptors also leads to ion level changes (primarily Ca2+), but these changes are not as rapid. Their amplitude and dynamics are much lower than the above. None of the compounds showed activity in barium chloride- or calcium chloride-induced arrhythmias. Therefore, we can assume that the blockade of sodium or calcium channels was not the predominant mechanism of antiarrhythmic effect of the studied compounds.
We decided to test therapeutic antiarrhythmic potential of the most active compounds (i.e., HBK-16, HBK-17, HBK-18, and HBK-19) in adrenaline-induced model of arrhythmia. The studied compounds restored normal heart rhythm administered at the peak of arrhythmia, but the strongest therapeutic antiarrhythmic activity showed HBK-18. The results of this experiments correlate with the results obtained in prophylactic adrenaline-induced arrhythmia model.
Antiarrhythmic agents have proarrhythmic potential, thus we evaluated the influence of studied compounds on normal ECG in rats. Only HBK-14 and HBK-15 did not influence ECG at ED84 obtained in prophylactic adrenaline-induced arrhythmia model. The rest of compounds significantly decreased heart rate. Williamson et al. (1994) proved that stimulation of α1A-adrenoceptors resulted in positive chronotropic effect. This suggests that the decrease in heart rate observed after treatment with HBK-16, HBK-17, HBK-18, and HBK-19 was a result of α1A receptor blockade.
The QT interval represents electrical depolarization and repolarization of ventricles. The prolongation of QT interval indicates the potential of a drug to cause ventricular tachyarrhythmias like torsades de pointes. The QTc adjusts the QT interval for heart rate extremes. In this study we used Bazett's equation to calculate QTc. Nevertheless, we need to point out that even though Bazett's formula is very often used for QT correction, it has many limitations (e.g., over- and under-correction of high or low heart rhythms). Rodents' heart rate values can be several times higher than those observed in humans (Kmecova and Klimas, 2010). Since heart rhythm significantly influences QTc, this might be the reason for the observed differences in baseline QTc in our experiments. We showed that none of the compounds affected QTc at ED84, therefore we can assume that they did not have proarrhythmic potential at antiarrhythmic doses.
Our findings are in agreement with the results obtained by other researchers, showing that phenylpiperazine derivatives possessed α1-adrenolytic properties, as well as prophylactic and/or therapeutic activity in adrenaline-induced arrhythmia (Dylag et al., 2004; Handzlik et al., 2012; Kubacka et al., 2013a,b).
When discussing adrenaline-induced arrhythmias we could neglect the role of α1D-adrenoceptors, since their blockade should not have a direct influence on cardiac myocytes. Nevertheless, in animal studies on drug candidates, we cannot entirely ignore the role of α1D-subtype. α1D-Adrenoceptors blockade in blood vessels might significantly lower blood pressure, which due to the baroreflex might increase heart rate, and contribute to arrhythmia.
Since all studied compounds blocked α1D-adrenoceptor, and these receptors among others regulate blood pressure, we evaluated their influence on blood pressure in rats. All tested compounds showed hypotensive properties. HBK-18 showed the strongest hypotensive activity, while HBK-15 the weakest. Interestingly, the results of this experiment did not correlate with the functional bioassays, where the strongest α1D-adrenoceptor blocking properties showed HBK-16. We suspect that this may be due to the differences in receptor binding dynamics, but this issue would require further experiments. Regarding the case of HBK-19, the lowest dose that reduced blood pressure was around three-fold higher than ED50 value in prophylactic adrenaline-induced arrhythmia. For antiarrhythmic drugs, hypotensive activity is not desirable, since α1-adrenoceptor blockers acting in the periphery, may induce reflex tachycardia, and contribute to cardiac arrhythmias. In our opinion, the lack of hypotensive properties at antiarrhythmic doses makes HBK-19 the most interesting compound in the studied group. Our results suggest that the receptor profile of HBK 19 (the highest affinity for α1A and the lowest for α1D) is the most beneficial in preventing adrenaline-induced arrhythmia. Interestingly, this suggests that in in vivo conditions the selectivity between α1A and α1D is much more important in achieving the optimal profile of α1-adrenolytics acting as antiarrhythmic agents, than the selectivity between α1A and α1B.
In order to prove that hypotensive properties of tested compounds were a result of their α1-adrenolytic properties, we performed the experiments with methoxamine (selective α1-adrenoceptor agonist). Drugs that selectively block α1-adrenergic receptors significantly inhibit pressor response to methoxamine. All studied compounds blocked the effect caused by methoxamine, thus we concluded that their hypotensive activity was due to α1-adrenolytic properties.
Oxidative stress plays an important role in arrhythmias (Dudek et al., 2014; Sovari, 2016). Reactive oxygen species (ROS) prolong action potential duration, induce early afterdepolarizations, and delayed afterdepolarizations in rats and guinea-pigs (Beresewicz and Horackova, 1991). Scientists indicated that oxidative stress activated Ca2+/CaM-dependent kinase II (CaMKII), and consequently caused arrhythmias (Xie et al., 2009). Rabbits with cardiac hypertrophy pretreated with CaMKII inhibitor were less likely to develop ventricular arrhythmias (Ke et al., 2007). Kirshenbaum et al. (1990) showed that vitamin E (antioxidant) protected rats with chronic heart hypertrophy against adrenaline-induced arrhythmias. This suggests that oxidative stress plays role in adrenaline-induced arrhythmias.
Given the significant antiarrhythmic effect of the studied compounds, we decided to investigate whether 2-methoxyphenylpiperazine derivatives possess additional antioxidant activity. Strong antioxidant activity might have contributed to their antiarrhythmic effect. This would explain their significant effect in adrenaline-induced model of arrhythmia. Our experiments showed that among all studied compounds only HBK-16, HBK-17, HBK-18, and HBK-19 weakly inhibited lipid peroxidation. The effect elicited by HBK-19 was the strongest in the group. However, its activity was around eight-fold weaker than the effect caused by carvedilol. Although HBK-16, HBK-17, HBK-18, and HBK-19 possessed weaker antioxidant properties than carvedilol, they showed stronger antiarrhythmic activity. This confirms that in adrenaline-induced arrhythmia model, the blockade of α1-adrenoceptors is more important for antiarrhythmic activity than antioxidant properties of the compound. Moreover, our findings suggest that antiarrhythmic properties of studied compounds resulted predominantly from α1-adrenolytic properties.
The levels of cardiac α1-adrenoceptor are around 10-fold higher in rats than in humans. This may suggest that the role of α1-adrenoceptor blockade in arrhythmia is not as significant in humans. Interestingly, despite lower expression of α1-adrenoceptors in human heart, scientists proved that they play a significant role in arrhythmias (Furushima et al., 2001). Kurtzwald-Josefson et al. (2014) identified a contribution of α-adrenergic pathway to pathogenesis of catecholamine-induced arrhythmia, and suggested α-blockade as an effective therapy in the murine model of catecholaminergic polymorphic ventricular tachycardia. The Authors suggested α-adrenolytics as an alternative treatment in humans resistant to β-blockers. Thus, it would be reasonable to keep searching for antiarrhythmic agents among α1-adrenolytics.
Since structural similarity of studied compounds reduces the likelihood of various mechanisms of action, in future studies we plan to investigate another set of structurally similar 2-methoxyphenylpiperazine derivatives with higher selectivity toward α1A-adrenoceptor subtype. This might give more insight into the role of α1A-adrenoceptor subtype in antiarrhythmic effect.
In conclusion, the studied 2-methoxyphenylpiperazine derivatives possessed high affinity for α1-adrenoceptors and competitively antagonized α1A, α1B, and α1D receptor subtypes. The compounds that possessed stronger α1A-adrenolytic properties (i.e., HBK-16, HBK-17, HBK-18, and HBK-19) were the most active compounds in adrenaline-induced arrhythmia. We suggest that their antiarrhythmic activity results predominantly from strong α1A-adrenolytic properties.
Author contributions
Conceived and designed the experiments: KP, SM, JS, BF. Performed the experiments: KP, SM, KL, EŻ, MK, AS, AD, JŚniecikowska, MZ. Analyzed the data: KP, SM, KL, EŻ, AO, AG, JŚmieja. Contributed reagents/materials/analysis tools: AW, HM. Wrote the paper: KP, SM, AO, KL, EŻ, MK.
Funding
This study was supported by Jagiellonian University grants number K/DSC/000040 and K/DSC/001955. This work was partially supported by NCN grant DEC- 2013/11/B/ST7/01713 and Silesian University BK grant 227/RAu1/2015/1.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank Agnieszka Niedbał and Teresa Dobrut for their technical assistance.
References
- Arunlakshana O., Schild H. O. (1959). Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 14, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresewicz A., Horackova M. (1991). Alterations in electrical and contractile behavior of isolated cardiomyocytes by hydrogen peroxide: possible ionic mechanisms. J. Mol. Cell. Cardiol. 23, 899–918. [DOI] [PubMed] [Google Scholar]
- Chalothorn D., McCune D. F., Edelmann S. E., Tobita K., Keller B. B., Lasley R. D., et al. (2003). Differential cardiovascular regulatory activities of the alpha 1B- and alpha 1D-adrenoceptor subtypes. J. Pharmacol. Exp. Ther. 305, 1045–1053. 10.1124/jpet.102.048553 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. (1973). Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108. [DOI] [PubMed] [Google Scholar]
- De Clerck F., Van de Water A., D'Aubioul J., Lu H. R., van Rossem K., Hermans A., et al. (2002). In vivo measurement of QT prolongation, dispersion and arrhythmogenesis: application to the preclinical cardiovascular safety pharmacology of a new chemical entity. Fundam. Clin. Pharmacol. 16, 125–140. 10.1046/j.1472-8206.2002.00081.x [DOI] [PubMed] [Google Scholar]
- Deo R., Albert C. M. (2012). Epidemiology and genetics of sudden cardiac death. Circulation 125, 620–637. 10.1161/CIRCULATIONAHA.111.023838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek M., Knutelska J., Bednarski M., Nowiński L., Zygmunt M., Bilska-Wilkosz A., et al. (2014). Alpha lipoic acid protects the heart against myocardial post ischemia-reperfusion arrhythmias via KATP channel activation in isolated rat hearts. Pharmacol. Rep. 66, 499–504. 10.1016/j.pharep.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Dylag T., Zygmunt M., Maciag D., Handzlik J., Bednarski M., Filipek B., et al. (2004). Synthesis and evaluation of in vivo activity of diphenylhydantoin basic derivatives. Eur. J. Med. Chem. 39, 1013–1027. 10.1016/j.ejmech.2004.05.008 [DOI] [PubMed] [Google Scholar]
- Eltze M. (1996). Functional evidence for an alpha 1B-adrenoceptor mediating contraction of the mouse spleen. Eur. J. Pharmacol. 311, 187–198. [DOI] [PubMed] [Google Scholar]
- Eltze M., König H., Ullrich B., Grebe T. (1999). Buspirone functionally discriminates tissues endowed with alpha1-adrenoceptor subtypes A, B, D and L. Eur. J. Pharmacol. 378, 69–83. [DOI] [PubMed] [Google Scholar]
- Escobar A. L., Perez C. G., Reyes M. E., Lucero S. G., Kornyeyev D., Mejía-Alvarez R., et al. (2012). Role of inositol 1,4,5-trisphosphate in the regulation of ventricular Ca(2+) signaling in intact mouse heart. J. Mol. Cell. Cardiol. 53, 768–779. 10.1016/j.yjmcc.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furushima H., Chinushi M., Washizuka T., Aizawa Y. (2001). Role of alpha1-blockade in congenital long QT syndrome: investigation by exercise stress test. Jpn. Circ. J. 65, 654–658. 10.1253/jcj.65.654 [DOI] [PubMed] [Google Scholar]
- Handzlik J., Bajda M., Zygmunt M., Maciąg D., Dybała M., Bednarski M., et al. (2012). Antiarrhythmic properties of phenylpiperazine derivatives of phenytoin with α1-adrenoceptor affinities. Bioorg. Med. Chem. 20, 2290–2303. 10.1016/j.bmc.2012.02.009 [DOI] [PubMed] [Google Scholar]
- Handzlik J., Maciag D., Kubacka M., Mogilski S., Filipek B., Stadnicka K., et al. (2008). Synthesis, α1-adrenoceptor antagonist activity, and SAR study of novel arylpiperazine derivatives of phenytoin. Bioorg. Med. Chem. 16, 5982–5998. 10.1016/j.bmc.2008.04.058 [DOI] [PubMed] [Google Scholar]
- Harrison S. N., Autelitano D. J., Wang B.-H., Milano C., Du X.-J., Woodcock E. A. (1998). Reduced reperfusion–induced ins(1,4,5)P3 generation and arrhythmias in hearts expressing constitutively active α1b-adrenergic receptors. Circ. Res. 83, 1232–1240. [DOI] [PubMed] [Google Scholar]
- Hieble J. P. (2000). Adrenoceptor subclassification: an approach to improved cardiovascular therapeutics. Pharm. Acta Helv. 74, 163–171. 10.1016/S0031-6865(99)00030-8 [DOI] [PubMed] [Google Scholar]
- Jähnichen S., Eltze M., Pertz H. H. (2004). Evidence that alpha(1B)-adrenoceptors are involved in noradrenaline-induced contractions of rat tail artery. Eur. J. Pharmacol. 488, 157–167. 10.1016/j.ejphar.2004.02.020 [DOI] [PubMed] [Google Scholar]
- Ke J., Zhang C., Ma Y., Liu J., Zhang Q., Liu N., et al. (2007). [The effects of calmodulin kinase II inhibitor on ventricular arrhythmias in rabbits with cardiac hypertrophy]. Zhonghua Xin Xue Guan Bing Za Zhi 35, 33–36. 10.3760/j:issn:0253-3758.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Kirshenbaum L. A., Gupta M., Thomas T. P., Singal P. K. (1990). Antioxidant protection against adrenaline-induced arrhythmias in rats with chronic heart hypertrophy. Can. J. Cardiol. 6, 71–74. [PubMed] [Google Scholar]
- Kmecova J., Klimas J. (2010). Heart rate correction of the QT duration in rats. Eur. J. Pharmacol. 641, 187–192. 10.1016/j.ejphar.2010.05.038 [DOI] [PubMed] [Google Scholar]
- Koshimizu T.-A., Tsujimoto G., Hirasawa A., Kitagawa Y., Tanoue A. (2004). Carvedilol selectively inhibits oscillatory intracellular calcium changes evoked by human alpha1D- and alpha1B-adrenergic receptors. Cardiovasc. Res. 63, 662–672. 10.1016/j.cardiores.2004.05.014 [DOI] [PubMed] [Google Scholar]
- Kubacka M., Mogilski S., Filipek B., Marona H. (2013a). Antiarrhythmic properties of some 1,4-disubstituted piperazine derivatives with α1-adrenoceptor affinities. Eur. J. Pharmacol. 720, 237–246. 10.1016/j.ejphar.2013.10.021 [DOI] [PubMed] [Google Scholar]
- Kubacka M., Mogilski S., Filipek B., Marona H. (2013b). The hypotensive activity and alpha1-adrenoceptor antagonistic properties of some aroxyalkyl derivatives of 2-methoxyphenylpiperazine. Eur. J. Pharmacol. 698, 335–344. 10.1016/j.ejphar.2012.10.025 [DOI] [PubMed] [Google Scholar]
- Kurtzwald-Josefson E., Hochhauser E., Bogachenko K., Harun-Khun S., Katz G., Aravot D., et al. (2014). Alpha blockade potentiates CPVT therapy in calsequestrin-mutant mice. Heart Rhythm 11, 1471–1479. 10.1016/j.hrthm.2014.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Rosen M. R. (1993). Modulation of delayed afterdepolarisations by alpha 1 adrenergic receptor subtypes. Cardiovasc. Res. 27, 839–844. [DOI] [PubMed] [Google Scholar]
- Litchfield J. T., Wilcoxon F. (1949). A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96, 99–113. [PubMed] [Google Scholar]
- O'Connell T. D., Ishizaka S., Nakamura A., Swigart P. M., Rodrigo M. C., Simpson G. L., et al. (2003). The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J. Clin. Invest. 111, 1783–1791. 10.1172/JCI16100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parés-Hipólito J., Gómez-Zamudio J. H., Gallardo-Ortiz I. A., López-Guerrero J. J., Santamaría-Ortiz J., Ibarra M., et al. (2006). Selective agonists reveal alpha(1A)- and alpha(1B)-adrenoceptor subtypes in caudal artery of the young rat. Auton. Autacoid Pharmacol. 26, 371–378. 10.1111/j.1474-8673.2006.00380.x [DOI] [PubMed] [Google Scholar]
- Pönicke K., Heinroth-Hoffmann I., Brodde O.-E. (2002). Differential effects of bucindolol and carvedilol on noradenaline-induced hypertrophic response in ventricular cardiomyocytes of adult rats. J. Pharmacol. Exp. Ther. 301, 71–76. 10.1124/jpet.301.1.71 [DOI] [PubMed] [Google Scholar]
- Proven A., Roderick H. L., Conway S. J., Berridge M. J., Horton J. K., Capper S. J., et al. (2006). Inositol 1,4,5-trisphosphate supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J. Cell Sci. 119, 3363–3375. 10.1242/jcs.03073 [DOI] [PubMed] [Google Scholar]
- Pytka K., Partyka A., Jastrzębska-Więsek M., Siwek A., Głuch-Lutwin M., Mordyl B., et al. (2015). Antidepressant- and anxiolytic-like effects of new dual 5-HT1A and 5-HT7 antagonists in animal models. PLoS ONE 10:e0142499. 10.1371/journal.pone.0142499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapacz A., Pytka K., Sapa J., Kubacka M., Filipek B., Szkaradek N., et al. (2014). Antiarrhythmic, hypotensive and α1-adrenolytic properties of new 2-methoxyphenylpiperazine derivatives of xanthone. Eur. J. Pharmacol. 735, 10–16. 10.1016/j.ejphar.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Rapacz A., Sapa J., Nowiński L., Mogilski S., Pytka K., Filipek B., et al. (2015a). Biofunctional studies of new 2-methoxyphenylpiperazine xanthone derivatives with α18281-adrenolytic properties. Pharmacol. Rep. 67, 267–274. 10.1016/j.pharep.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Rapacz A., Sapa J., Pytka K., Dudek M., Filipek B., Szkaradek N., et al. (2015b). Antiarrhythmic activity of new 2-methoxyphenylpiperazine xanthone derivatives after ischemia/reperfusion in rats. Pharmacol. Rep. 67, 1163–1167. 10.1016/j.pharep.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Sapa J., Filipek B., Nowiński L. (2011). Antiarrhythmic and hypotensive activities of 1-[2-hydroxy-3-(4-phenyl-1-piperazinyl)propyl]-pyrrolidin-2-one (MG-1(R,S)) and its enantiomers. Pharmacol. Rep. 63, 455–463. 10.1016/S1734-1140(11)70512-6 [DOI] [PubMed] [Google Scholar]
- Shannon R., Chaudhry M. (2006). Effect of alpha1-adrenergic receptors in cardiac pathophysiology. Am. Heart J. 152, 842–850. 10.1016/j.ahj.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Sovari A. A. (2016). Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol. Res. Pract. 2016, 1–7. 10.1155/2016/9656078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suita K., Fujita T., Hasegawa N., Cai W., Jin H., Hidaka Y., et al. (2015). Norepinephrine-induced adrenergic activation strikingly increased the atrial fibrillation duration through β1- and α1-adrenergic receptor-mediated signaling in mice. PLoS ONE 10:e0133664. 10.1371/journal.pone.0133664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres L., Papp J. G. (1968). Antiarrhythmic compounds. Prog. drug Res. Fortschritte der Arzneimittelforschung. Progrès des Rech. Pharm. 12, 292–369. [DOI] [PubMed] [Google Scholar]
- Szekeres L., Papp J. G. (1975). Experimental cardiac arrhythmias, in Experimental Production of Diseases, Part 3, Heart and Circulation, Handbook of Experimental Pharmacology, Vol. XVI/3, eds Schmier J., Eichler O. (New York, NY; Berlin; Heidelberg: Springer-Verlag; ), 131–182. [Google Scholar]
- Waszkielewicz A. M., Pytka K., Rapacz A., Wełna E., Jarzyna M., Satała G., et al. (2015). Synthesis and evaluation of antidepressant-like activity of some 4-substituted 1-(2-methoxyphenyl)piperazine derivatives. Chem. Biol. Drug Des. 85, 326–335. 10.1111/cbdd.12394 [DOI] [PubMed] [Google Scholar]
- Williamson A. P., Seifen E., Lindemann J. P., Kennedy R. H. (1994). Alpha 1a-adrenergic receptor mediated positive chronotropic effect in right atria isolated from rats. Can. J. Physiol. Pharmacol. 72, 1574–1579. [DOI] [PubMed] [Google Scholar]
- Xie L.-H., Chen F., Karagueuzian H. S., Weiss J. N. (2009). Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ. Res. 104, 79–86. 10.1161/CIRCRESAHA.108.183475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T. L., Cheng H. Y., Lysko P. G., McKenna P. J., Feuerstein R., Gu J. L., et al. (1992). Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J. Pharmacol. Exp. Ther. 263, 92–98. [PubMed] [Google Scholar]
- Zima A. V., Blatter L. A. (2004). Inositol-1,4,5-trisphosphate-dependent Ca(2+) signalling in cat atrial excitation-contraction coupling and arrhythmias. J. Physiol. 555, 607–615. 10.1113/jphysiol.2003.058529 [DOI] [PMC free article] [PubMed] [Google Scholar]