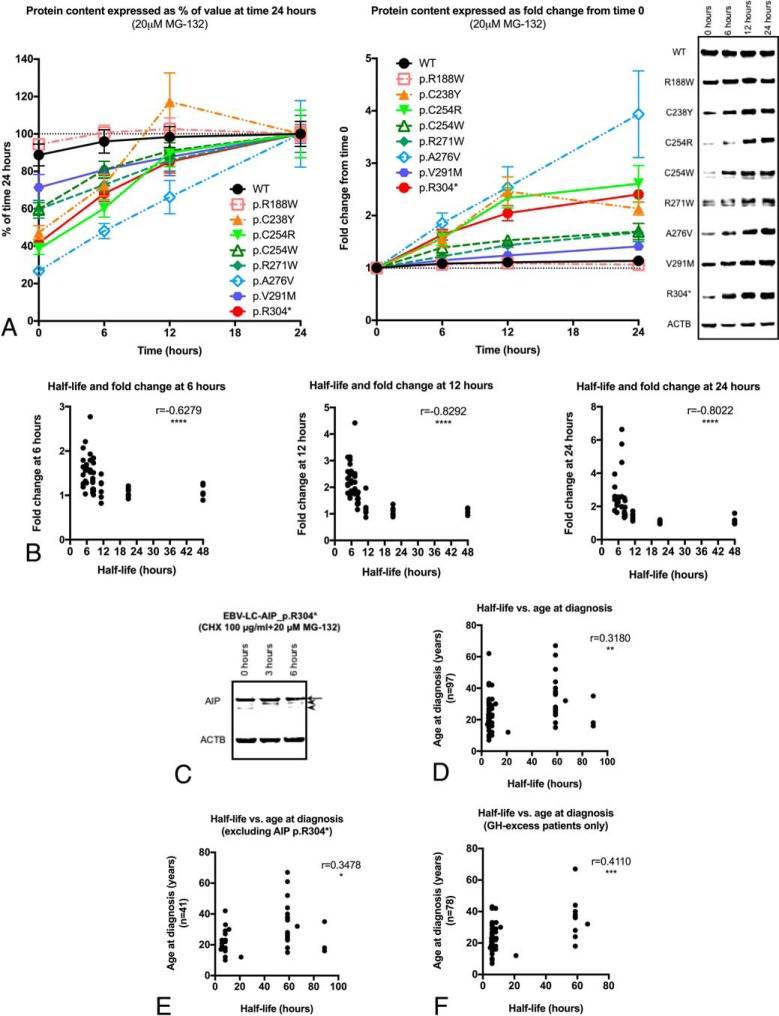
Figure 3.
Blocking of proteasomal degradation with MG-132 (“rescue experiments”) and implications of AIP half-life for the phenotype in pituitary adenoma patients. A, Curves of protein contents for the experiments with the variants and WT protein, expressed as percentages (level at the 24-h time point was considered as 100% for each protein) or as fold change from time 0 (time 0 = 1) and representative WB images. The AIP variants p.R188W (fold change at 24 h, 1.1; global P = .5080) and p.V291M (fold change at 24 h, 1.4; global P = .1263) were stable. In contrast, levels of the other variants studied displayed a significant rise in response to MG-132: p.C238Y (fold change, 1.6 [P = .0403]; 2.5 [P = .0170]; and 2.1 [P = .0015] at 6, 12, and 24 h, respectively), p.C254R (fold change, 1.6 [P = .0087]; 2.3 [P = .0008]; and 2.6 [P = .0094]), p.C254W (fold change, 1.4 [P = .0910]; 1.5 [P = .0006]; and 1.7 [P = .0087]), p.R271W (fold change, 1.4 [P = .1796]; 1.5 [P = .0229]; and 1.7 [P = .0676]), p.A276V (fold change, 1.9 [P = .0072]; 2.5 [P = .0120]; and 3.9 [P = .0225]) and p.R304* (fold change, 1.6 [P = .0055]; 2 [P = .0041]; and 2.4 [P < .0001]). Myc-AIP, 39 kDa; Myc-AIP p.R304*, 35.8 kDa; ACTB, 41.7 kDa. ACTB loading control shown for the WT experiment in each case. B, Correlation between half-life and fold change after MG-132 treatment at 6, 12, and 24 h. A significant indirect correlation was found at the three time points, indicating proteins with shorter half-lives responded better to proteasome inhibition. C, MG-132 treatment of EBV-LC-AIP_p.R304* cells. In the AIP WB (using 40 μg of total protein) the strongest band (arrow) corresponds to the WT protein (37.6 kDa). Two faint extra bands can be observed: a first band that is absent at time 0 and appears after the treatment with MG-132 (top arrowhead) and a second, lower band (bottom arrowhead), which is more evident at time 0. Although both bands could correspond to degradation products, the heaviest one could possibly be given by a small amount of truncated protein, appearing after proteasomal degradation is inhibited (expected size, 34.5 kDa). Loading control: ACTB (41.7 kDa). D, The half-life of the studied AIP variants directly correlated with the age at diagnosis, (E) even when excluding patients with the p.R304* mutant; (F) the significance increased when considering only patients with acromegaly or gigantism.