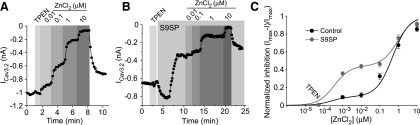
FIG. 6.
SP increases zinc-mediated inhibition of CaV3.2 that is mediated by a high-affinity binding site. (A) Exemplary patch-clamp recording from HEK293 cell co-transfected with CaV3.2 and NK1 showing concentration dependency of the effect of extracellular ZnCl2 on CaV3.2 currents. TPEN (10 μM) was applied at the beginning of the experiment to record CaV3.2 current amplitude at a near-zero extracellular zinc concentration. Increasing concentrations of ZnCl2 were applied during the periods indicated by vertical gray bars. (B) Experiment similar to (A) but increasing ZnCl2 concentrations were applied after application of 1 μM S9SP; 1 μM S9SP was present in all ZnCl2 solutions. (C). The data from 6–12 experiments as in (A, B) were fit to two-component logistic function (see Materials and Methods section). The solution containing 10 μM TPEN and no added ZnCl2 was assigned a free zinc concentration of 0.05 nM. In control conditions, the IC50(1) was 0.25 ± 1.4 nM and the IC50(2) was 495 ± 326 nM. After the S9SP treatment, the IC50(1) was 0.35 ± 0.25 nM and the IC50(2) was 923 ± 582 nM. (C) Data are shown as mean ± SEM. HEK, human embryonic kidney.