Abstract
Objectives: Clinically stable children with HIV can have neuromotor, attention, memory, visual–spatial, and executive function impairments. We evaluated neuropsychological and behavioral benefits of computerized cognitive rehabilitation training (CCRT) in Ugandan HIV children.
Design: One hundred fifty-nine rural Ugandan children with WHO Stage I or II HIV disease (6 to 12 years; 77 boys, 82 girls; M = 8.9, SD = 1.86 years) were randomized to one of three treatment arms over a 2-month period.
Methods: The CCRT arm received 24 one-hour sessions over 2 months, using Captain's Log (BrainTrain Corporation) programmed for games targeting working memory, attention, and visual–spatial analysis. These games progressed in difficulty as the child's performance improved. The second arm was a “limited CCRT” with the same games rotated randomly from simple to moderate levels of training. The third arm was a passive control group receiving no training. All children were assessed at enrollment, 2 months (immediately following CCRT), and 3 months after CCRT completion.
Results: The CCRT group had significantly greater gains through 3 months of follow-up compared to passive controls on overall Kaufman Assessment Battery for Children–second edition (KABC-II) mental processing index (p < .01), planning (p = .04), and knowledge (p = .03). The limited CCRT group performed better than controls on learning (p = .05). Both CCRT arms had significant improvements on CogState Groton maze learning (p < .01); although not on CogState attention/memory, TOVA/impulsivity, or behavior rating inventory for executive function and child behavior checklist (psychiatric behavior/symptom problems) ratings by caregiver.
Conclusions: CCRT intervention can be effective for neurocognitive rehabilitation in children with HIV in low-resource settings, especially in children who are clinically stable on ARV treatment.
Introduction
While initial focus in pediatric HIV was rightly placed on improved survival,1,2 there is increasing need to focus on the quality of life for African children living with HIV. Cognitive, psychiatric, and behavioral (neuropsychological) disorders are emerging as a major concern in highly active antiretroviral therapy (HAART)-treated perinatally infected children as they progress into adolescence.3,4 HIV disease in children can significantly impair attention, memory, and visual–spatial processing speed,5–9 impairing school performance. These are the very domains of neurocognitive development in African children that have been most effectively improved through computerized cognitive rehabilitation treatment, computerized cognitive rehabilitation training (CCRT).10
Preliminary studies of the neurocognitive benefits of CCRT with Captain's Log in Ugandan Children with HIV documented generalization improvements on standardized tests of simple attention and maze learning.11 Similar improvements were noted for Captain's Log CCRT with children surviving severe malaria.12,13 Although CCRT had an immediate effect on cognitive outcomes pertaining to attention and some aspects of learning, the evidence was inconclusive in terms of neuropsychological or psychosocial behavioral benefit at school or in the home.
In addition, although a hallmark feature of CCRT procedures is to titrate difficulty of training with ongoing performance, it was unclear how this individualized titration would compare to computer games without this component. A recent report comparing working memory (WM) CCRT with and without performance titration concluded that titration is important for neuroconnectivity to take place in underlying brain regions leading to improved neurocognitive performance.14 This study also compares CCRT with and without titration in the context of a clinical trial and includes a third group that does not receive CCRT. This took place with rural children in Uganda in an impoverished region where the HIV/AIDS epidemic has been most devastating and children face a myriad of accompanying risk factors to their neuropsychological development.15,16
Methods
Participants
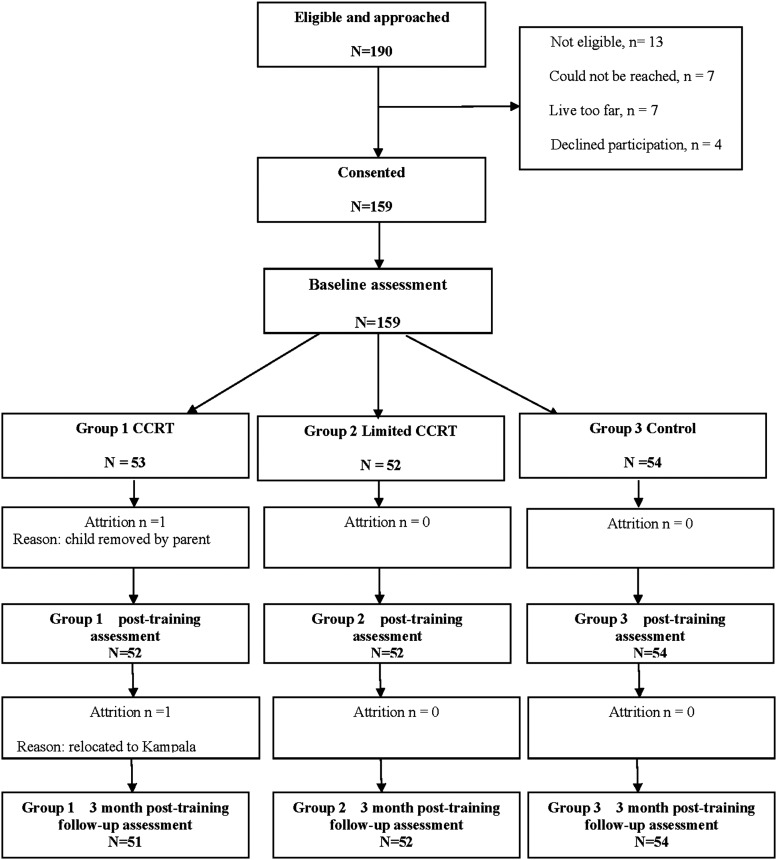
One hundred fifty-nine rural Ugandan children with WHO Stage I or II HIV disease (6 to 12 years; 77 boys, 82 girls; M = 8.9, SD = 1.86 years) were randomly assigned using a random numbers table by the study manager to one of three treatment arms over a 2-month period. The CCRT arm received 24 one-hour sessions (3 days per week) using Captain's Log (BrainTrain Corporation) programmed for games targeting WM, attention, and visual–spatial analysis. This is the recommended amount of training to achieve a significant neurocognitive benefit.17–19 The second arm was a “limited CCRT” with the same nine games rotated randomly through the simplest to moderate levels of training (no titration). The third arm was a passive control group receiving no computer training. All children were assessed at enrollment, 2 months (post-CCRT), and 3 months after CCRT (Fig. 1), at a private study clinic in Kayunga.
FIG. 1.
CONSORT diagram of flow of participants through clinical trial, including sample selection, randomization to treatment arms, retention, and outcomes assessment number for final analyses. CCRT, computerized cognitive rehabilitation training.
Assessments were conducted in the local language of the children (Luganda) by native speakers. Assessors were unaware of the child's treatment arm. Socioeconomic status (SES) and quality of home environment [home observation for the measurement of the environment (HOME)] was assessed upon enrollment. Children had access to daily medical care and ART was initiated as per Uganda country 2008 guidelines.20 Informed written consent was obtained from the child's parent and signed assent from children age >7 years. Consent and assent forms were read to the parent and child in the local language. IRB approval for this study was obtained from Makerere University School of Medicine, University of Michigan, and Michigan State University. Research permission was obtained from the Uganda National Council for Science and Technology. Enrollment, assessment, and training took place from 2011 to 2013.
Computerized cognitive rehabilitation training
Captain's Log CCRT intervention
Captain's Log® marketed by BrainTrain Corporation, is a comprehensive set of computerized cognitive training games to improve a wide range of cognitive skills.21 Research assistants supervising CCRT spoke the local language of Luganda when instructing the children on the training tasks. Training was done in the child's home after school so as to avoid bringing unnecessary attention to the child and possible stigmatization by classmates. We used the same configuration of Captain's Log that was used in our preliminary studies.10,11 A Uganda psychology team led by M.J.B. and B.G. reviewed each of the possible training tasks and selected nine games considered to be most culturally fair, with three tasks emphasizing visual–spatial WM, vigilance attention, and nonverbal reasoning, respectively.
All children were started at the simplest program level for each task with training becoming more difficult as children progressed. Sessions lasted 45 to 60 min. Captain's Log has an internal evaluator (CLIE) feature, which can be used to audit the child's rate and level of progress in each session, while advancing to progressively more difficult items. We used this feature to assess fidelity of CCRT.
Limited Captain's Log CCRT
For the limited CCRT group, we programmed Captain's Log so that it rotated randomly from simple to moderate levels of training for the entire session. In every other respect, this training arm was like the full CCRT arm.
Assessments for neuropsychological outcomes
Kaufman Assessment Battery for Children–second edition
The Kaufman Assessment Battery for Children–second edition (KABC-II) measures cognitive abilities in children aged 3 to 18 years and is designed to minimize the influence of language and cultural knowledge on test results.22 A special Nonverbal Index, used in this study, allows children to be tested using only gestures to communicate instructions and requires no understanding of English or the need to engage in spoken verbal response. The KABC-II maintained its factor structure with Ugandan cerebral malaria survivors23 and is sensitive to clinical biomarkers of disease status in Ugandan children with HIV.9,24 The primary outcome variables were the global scores of Sequential Processing (memory), Simultaneous Processing (visual–spatial analysis), Learning (immediate and delayed memory), Planning (executive reasoning), and Knowledge (crystallized intelligence). These (except for Knowledge) are combined into a total mental processing index (MPI).
CogState
This computerized neurocognitive assessment was used in our prior CCRT studies.13,25–27 A 30-min session is presented, which includes playing cards in a game-like manner to assess memory, attention, discrimination learning, and executive function that is nonlanguage dependent. It does so with card detection (simple reaction time), identification (choice reaction time), one-back WM, and one-card learning. It also includes the Groton Maze Task that includes components to measure visual-motor tracking (Maze Chase) and executive functioning/planning (Maze Learning).
Test of variables of attention
The test of variables of attention (TOVA) is a computer-based measure of sustained attention and impulsivity.28 This test takes about 23 min to administer and is designed to minimize cultural differences, and has been used in Uganda to assess cognitive factors associated with pediatric HIV.9,24 The TOVA rapidly presents simple geometric stimuli (i.e., a large open square with either a solid smaller square at the top or bottom of the open square for each presentation) to which the child must respond by pressing a hand switch. Performance outcomes include percent trials with omissions errors of inattention (missing the correct target), percent trials with commissions errors of impulsivity (identifying the incorrect target as correct), mean response time (speed of processing), and variability in response times (consistency of attention processing). Summary performance measures include an ADHD index score [ranging from −10 (High ADHD) to positive 10 (Highly attentive)] and a D prime signal detection measure (sensitivity measures based on correct hits and correct misses).
Captain's Log performance measures
The Captain's Log internal evaluator (CLIE) measures of session performance were used to gauge fidelity of training during a training session.21 These measure a child's success at correctly responding to all training items for that session, accuracy of responses, speed of processing, and processing speed for correct responses to training items. These measures were entered into the database for all 24 training sessions, and improvements in the CLIE were correlated with improvements in other neuropsychological and behavioral outcomes for the CCRT and limited CCRT treatment arm children.
Bruininks/Oseretsky test for motor proficiency–second edition
This is a comprehensive and sensitive instrument for pediatric motor proficiency.29 Testing involves game-like tasks that hold the child's interest and are not verbally complex. Composite scores include fine manual control, manual coordination, body coordination, strength and agility, and total composite score. Its use in this study is particularly relevant because of the pronounced motor impairment that seems to accompany HIV-related neurocognitive delay compared with impairment from severe malaria.30,31
Behavior rating inventory for executive function
The behavior rating inventory for executive function (BRIEF) was developed to especially evaluate behavioral and cognitive problems as they relate to disruption of executive functions of the brain resulting from mild brain injury and/or developmental disorders. The BRIEF has been translated into the local language of Luganda and structure validated for use with this sample of children.33 It is also sensitive to the clinical status of children in our sample.34 The BRIEF school-age form was used for all children and takes ∼20–30 min to complete by the parent or principal caregiver. The eight nonoverlapping clinical scales form two broader indexes, Behavior Regulation (three scales) and Metacognition (five scales), and a Global Executive Composite score.
Achenbach child behavior checklist (parent)
We chose the child behavior checklist (CBCL) for our psychiatric assessment measure because it is one of the most widely used screening tools in child and adolescent psychiatry and pediatrics and has been translated into the local language of Luganda and structure validated for use with this sample of children.33 It has been previously used by our group in pediatric HIV research in Uganda.35,36 Although the CBCL can provide a wide array of DSM-V and Syndrome scale scores, our principal outcomes were Internalizing, Externalizing, and Total symptoms.
Control variables
Outcome analyses were all adjusted for age, gender, physical growth, socioeconomic status, quality of home environment score, recruitment location, and HAART status (yes/no), based on past research within the Sub-Saharan African context indicating the importance of both basic demographic factors, as well as home environment and occupation/income.37 The middle childhood version of the Caldwell HOME38,39 was used to assess the stimulation and learning opportunities offered by the child's home environment. Along with the HOME, we also used a socioeconomic evaluation scale of physical quality of the home environment (SES score) previously validated with Ugandan children as sensitive to long-term neurocognitive outcomes.40
CD4+ T-cell counts, immune activation, and viral load testing
CD4, CD8, viral load, and other immunology measures were available from a blood draw taken within a week from the time of neuropsychological assessment. CD4 T-cell and CD8 T-cell activation level testing was performed on EDTA anticoagulated whole blood using a fresh lyse no-wash flow cytometry procedure. Blood was incubated with monoclonal antibodies, including CD3, CD8, CD4, CD38, and HLA DR, then processed and acquired on a multilaser benchtop flow cytometer. For each run of patient samples, a separate sample of stabilized blood product (CD-Chex; Streck Laboratories) was processed. CD4 T-cell and CD8 T-cell activation levels were defined as % CD38 and HLA-DR coexpression.41
Statistical analyses
Adjusted means of the outcome variables were obtained from linear mixed-effects (LME) models with two repeated measures: (1) immediately after CCRT (2 months after intake into the trial) and (2) 3 months following completion of CCRT. Baseline measures of the outcome variables were used as covariates for added control of any preintervention differences among trial arms. The success of randomization was checked by comparing baseline variables by trial arm using the analyses of variance or chi-square tests, as appropriate. Variables that differed by trial arm at baseline were included as covariates in later analyses. The repeated measures analyses were also adjusted for the control variables. The primary analysis focused on the main (additive) effect of the trial arm. The average differences among trial arms over time were reflected by the adjusted (least square) means that were output from the LME model for each outcome variable. Additional analyses assessed the fidelity of the intervention by correlating Captain's Log internal success measures with postintervention outcomes. Finally, to explore whether the effect of the intervention differed according to HIV immunological measures or HAART status, the interactions of the trial arm with these measures were added (one at a time) to the LME models.
Results
Table 1 provides descriptive statistical information for these children following enrollment and before CCRT. Significantly fewer children were on HAART at the start of the study for the Limited CCRT arm, and they also had significantly higher KABC-II Learning and Delayed Recall scores related to Learning. Therefore, subsequent comparisons on neuropsychological outcomes between trial arms included these measures at baseline as covariates. There were no significant changes between posttraining and 3-month follow-up performance on any of our neuropsychological or behavioral outcome measures; so these were combined in comparing differences among trial arms for all of the LME analyses reported below.
Table 1.
Demographic Characteristics and Outcomes at Baseline by Trial Arm
| Characteristic | CCRT (N = 53), n (%) | Limited CCRT (N = 52), n (%) | Control (N = 54), n (%) | p-Value for comparison by arm |
|---|---|---|---|---|
| Sex | ||||
| Male | 29 (55) | 26 (49) | 22 (42) | .39 |
| Female | 24 (45) | 27 (51) | 31 (58) | |
| County | ||||
| Kayunga (periurban) | 6 (11) | 16 (30) | 13 (25) | .13 |
| Kangulumira (rural) | 11 (21) | 7 (13) | 12 (22) | |
| Other (rural) | 36 (68) | 30 (57) | 28 (53) | |
| Caregiver | ||||
| Mother | 23 (43) | 20 (38) | 18 (34) | .60 |
| Other | 30 (57) | 33 (62) | 35 (66) | |
| On HAART at intake | ||||
| No | 27 (51) | 15 (28) | 25 (47) | .04* |
| Yes | 26 (49) | 38 (72) | 28 (53) | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
|---|---|---|---|---|
| Age in years | 9.20 (1.70) | 8.86 (1.90) | 8.65 (1.97) | .30 |
| Weight-for-age z scorea | −1.27 (0.81) | −1.14 (1.01) | −1.17 (0.93) | .74 |
| Height-for-age z scorea | −1.19 (1.42) | −1.28 (1.44) | −1.22 (1.34) | .94 |
| Socioeconomic score | 9.68 (3.10) | 11.00 (3.50) | 10.04 (2.93) | .09 |
| HOME score total | 28.19 (6.71) | 29.87 (6.70) | 29.34 (5.85) | .40 |
| CD4% | 35.61 (14.06) | 37.25 (15.47) | 34.92 (15.22) | .71 |
| CD4_38H (CD4 activation) | 5.12 (4.53) | 5.23 (5.46) | 6.09 (5.01) | .56 |
| CD8% | 54.14 (13.23) | 52.84 (14.27) | 54.15 (13.44) | .85 |
| CD8_38H (CD8 activation) | 17.00 (10.35) | 14.84 (10.69) | 18.21 (12.01) | .29 |
| Viral load (log) | 7.21 (5.47) | 5.72 (5.54) | 7.66 (5.32) | .17 |
| KABC-IIb sequential processing | 69.25 (9.95) | 70.25 (8.70) | 68.38 (10.22) | .61 |
| KABC-II simultaneous processing | 59.85 (9.55) | 60.81 (11.19) | 61.96 (9.88) | .83 |
| KABC-II learning | 64.01 (9.14) | 71.00 (11.31) | 64.81 (8.56) | <.01** |
| KABC-II knowledge | 63.62 (10.35) | 64.85 (13.62) | 62.13 (13.10) | .53 |
| KABC-II planning | 59.23 (7.07) | 58.61 (11.74) | 59.90 (7.59) | .81 |
| KABC-II delayed recall | 65.89 (8.50) | 70.77 (10.78) | 67.17 (7.95) | .02* |
| KABC-II nonverbal index | 57.68 (7.96) | 58.23 (13.54) | 58.36 (7.62) | .94 |
| KABC-II mental processing index | 56.83 (7.16) | 59.30 (8.50) | 57.91 (6.54) | .23 |
| TOVA% omission errors | 22.81 (20.39) | 21.53 (21.13) | 26.55 (20.81) | .44 |
| TOVA% commission errors | 12.06 (10.77) | 13.32 (13.46) | 13.07 (11.12) | .85 |
| TOVA response time (msec) | 651.81 (146.62) | 653.42 (140.53) | 692.77 (143.92) | .25 |
| TOVA response time variability (msec) | 251.43 (72.02) | 254.30 (82.27) | 270.71 (67.41) | .35 |
| TOVA D prime signal detection score | 2.42 (0.95) | 2.28 (1.18) | 2.06 (1.03) | .52 |
| TOVA ADHD index | −6.09 (3.15) | −5.35 (3.25) | −5.99 (2.45) | .38 |
| BOT-2 total motor scorec | 33.06 (4.40) | 32.34 (5.24) | 31.79 (5.40) | .43 |
| CogState, maze chase correct moves per second | 0.20 (0.13) | 0.22 (0.14) | 0.19 (0.13) | .46 |
| CogState, moves maze learning correct moves per second | 0.10 (0.07) | 0.09 (0.08) | 0.07 (0.08) | .07 |
| CogState, detection time: playing card turning (log msec) | 2.85 (0.11) | 2.83 (0.12) | 2.86 (0.10) | .32 |
| CogState, identification time: red playing card turning log msec) | 2.98 (0.10) | 2.98 (0.10) | 3.00 (0.09) | .73 |
| CogState, accuracy in one-card learning | 0.62 (0.18) | 0.60 (0.14) | 0.57 (0.16) | .26 |
| CogState, accuracy in one-back card memory | 0.67 (0.29) | 0.70 (0.27) | 0.58 (0.28) | .10 |
| BRIEF behavior regulation indexc | 47.83 (11.13) | 46.09 (9.09) | 44.79 (7.12) | .28 |
| BRIEF metacognition index | 50.04 (11.31) | 47.00 (8.62) | 49.13 (9.83) | .32 |
| BRIEF global executive composite | 49.17 (11.13) | 46.45 (8.12) | 47.44 (8.27) | .36 |
| CBCL externalizing totalc | 60.69 (9.92) | 58.42 (6.88) | 58.25 (7.52) | .24 |
| CBCL internalizing total | 61.63 (8.73) | 60.13 (8.63) | 60.26 (10.23) | .65 |
| CBCL total | 60.00 (9.53) | 57.25 (7.52) | 58.13 (8.25) | .31 |
WHO 2013 norms.
Standardized scores using age-based norms.
T scores using age- and gender-based norms.
p < .05; **p < .01.
p-Values in bold are statistically significant.
BOT-2, Bruininks/Oseretsky test for motor proficiency–second edition; BRIEF, behavior rating inventory for executive function; CBCL, child behavior checklist; CCRT, computerized cognitive rehabilitation training; HAART, highly active antiretroviral therapy; HOME, home observation for the measurement of the environment; KABC-II, Kaufman Assessment Battery for Children–second edition; TOVA, test of variables of attention.
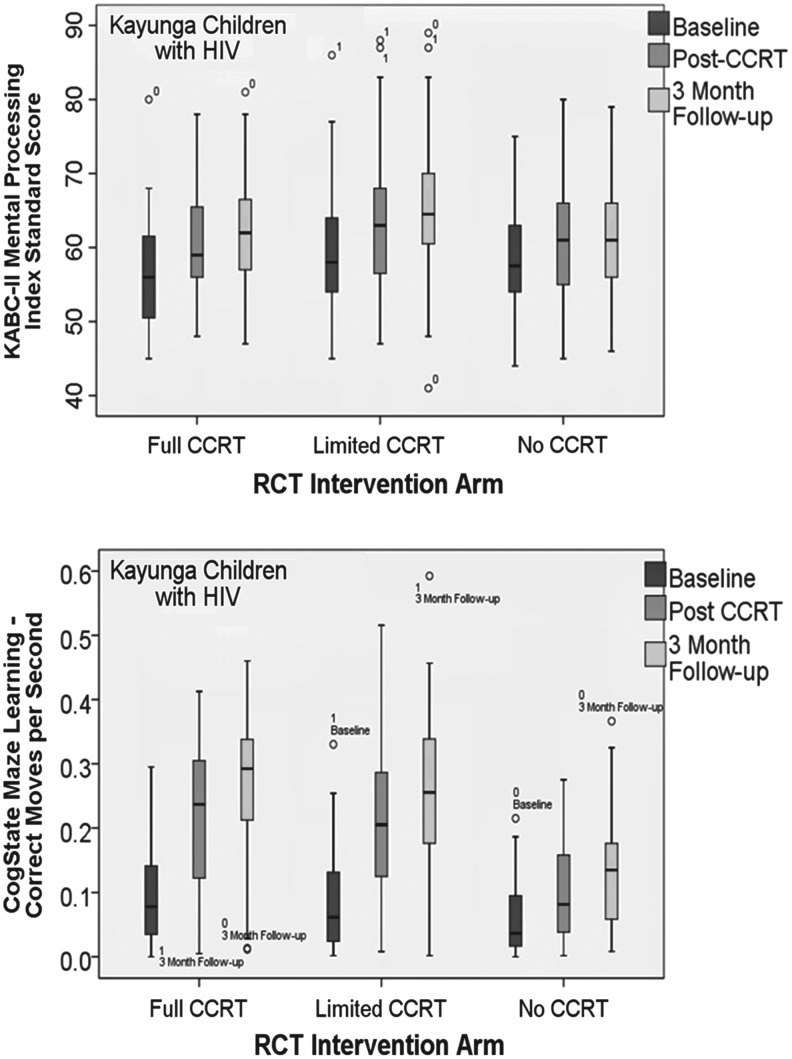
At posttraining and 3-month follow-up (Table 2), the CCRT group had significantly greater gains compared to passive controls on overall KABC-II performance (MPI, p < .01; Fig. 2), Planning (p = .04), and Knowledge (p = .03). The limited CCRT group performed better than controls on Learning (p = .05). Although marginal (p < .06), the CCRT group did not reach significance in terms of improvement on Learning. Both CCRT arms had significantly greater gains on CogState Groton maze chasing, on learning (p < .01; Fig. 2), and on card detection (p = .05). However, there were no treatment arm differences on any of the other CogState memory or attention measures, TOVA/impulsivity, or BRIEF and CBCL behavior/symptom ratings. In Table 3, significant interaction effects evidenced that children on HAART at study enrollment showed more dramatic KABC-II (Learning; p < .05) and CogState (one-back card learning, p < .05) performance benefit from the CCRT treatment arms that were not previously evident (Table 3).
Table 2.
Least Square Means from Longitudinal Model, Their Standard Errors by Trial Arm Adjusted for Age, Being on HAART at Intake, Socioeconomic Score, Home Score, Recruitment Location, KABC Learning and Delayed Recall Scores at Baseline, and Outcome Score at Baseline
| Neuropsychology outcomes | CCRT LS mean (SE) | Limited CCRT LS mean (SE) | Control LS mean (SE) | p-Value for comparison by arm | p-Value for CCRT vs. control | p-Value for limited CCRT vs. control |
|---|---|---|---|---|---|---|
| KABC-II sequential processinga | 73.00 (0.97) | 73.26 (0.99) | 72.77 (0.91) | .93 | .86 | .71 |
| KABC-II simultaneous processing | 69.18 (1.15) | 67.35 (1.19) | 66.20 (1.09) | .14 | .05 | .47 |
| KABC-II learning* | 72.23 (1.02) | 72.43 (1.05) | 69.66 (0.96) | .08 | .06 | .05 |
| KABC-II knowledge* | 70.32 (0.97) | 67.39 (1.00) | 67.72 (0.91) | .05 | .04 | .80 |
| KABC-II planning | 62.73 (0.74) | 62.50 (0.81) | 60.45 (0.77) | .07 | .03 | .07 |
| KABC-II delayed recall | 71.93 (0.94) | 71.02 (0.97) | 69.60 (0.89) | .17 | .06 | .27 |
| KABC-II nonverbal index | 63.67 (0.98) | 63.04 (1.01) | 61.26 (0.92) | .15 | .06 | .19 |
| KABC-II mental processing index** | 63.32 (0.60) | 62.65 (0.62) | 61.13 (0.57) | .02 | <.01 | .07 |
| TOVA% omission errors | 22.35 (2.05) | 23.18 (2.12) | 21.18 (1.94) | .77 | .67 | .48 |
| TOVA% commission errors | 10.93 (1.24) | 11.63 (1.28) | 9.67 (1.17) | .50 | .44 | .25 |
| TOVA response time (msec) | 651.19 (12.53) | 659.61 (12.92) | 654.32 (11.87) | .89 | .85 | .76 |
| TOVA response time variability (msec) | 249.06 (7.85) | 249.33 (8.09) | 246.62 (7.43) | .96 | .81 | .80 |
| TOVA D prime signal detection score | 2.33 (0.12) | 2.33 (0.13) | 2.49 (0.12) | .55 | .34 | .36 |
| TOVA ADHD index | −5.57 (0.40) | −6.09 (0.42) | −5.65 (0.38) | .61 | .87 | .43 |
| BOT-2 total motor scoreb | 34.63 (0.47) | 35.05 (0.47) | 34.35 (0.43) | .53 | .65 | .27 |
| CogState, maze chase** correct moves per second | 0.47 (0.02) | 0.48 (0.02) | 0.31 (0.02) | <.01 | <.01 | <.01 |
| CogState, maze learning** correct moves per second | 0.25 (0.01) | 0.23 (0.01) | 0.15 (0.01) | <.01 | <.01 | <.01 |
| CogState, detection time* card turned (log msec) | 2.83 (0.01) | 2.84 (0.01) | 2.86 (0.01) | .13 | .05 | .21 |
| CogState, identification red card turned (log msec) | 2.97 (0.01) | 2.98 (0.01) | 2.97 (0.01) | .65 | .81 | .50 |
| CogState, accuracy in one-card learning | 0.63 (0.02) | 0.60 (0.02) | 0.61 (0.02) | .36 | .31 | .67 |
| CogState, accuracy in one-back card memory | 0.74 (0.03) | 0.70 (0.03) | 0.74 (0.03) | .46 | .97 | .29 |
| BRIEF behavior regulation indexb | 45.05 (1.03) | 43.76 (1.07) | 44.90 (0.97) | .61 | .91 | .42 |
| BRIEF metacognition index | 46.42 (0.99) | 45.69 (1.03) | 45.44 (0.93) | .73 | .45 | .85 |
| BRIEF global executive composite index | 45.34 (0.97) | 44.87 (1.02) | 45.40 (0.90) | .91 | .97 | .69 |
| CBCL Externalizing Total | 56.77 (0.89) | 55.19 (0.92) | 54.88 (0.84) | .24 | .11 | .80 |
| CBCL Internalizing Total | 59.35 (0.92) | 56.69 (0.95) | 57.70 (0.87) | .11 | .18 | .42 |
| CBCL Total | 55.61 (0.83) | 53.46 (0.86) | 54.45 (0.78) | .18 | .29 | .38 |
Standardardized scores (using age-based norms).
T scores using age- and gender-based norms.
p < .05 for CCRT versus limited CCRT.
p < .01 for CCRT versus limited CCRT.
p-Values in bold are statistically significant.
SE, standard errors.
FIG. 2.
Box plots comparing RCT treatment arms (Full CCRT, Limited CCRT, No CCRT) on KABC-II mental processing index (upper graph; standardized score) and CogState maze learning task (lower graph; correct moves per second). Box represents median (bisect), upper and lower quartile, and range of values, as well as individual outliers.
Table 3.
KABC and CogState Outcomes of Those on HAART at Intake: Least Square Means from Longitudinal Model, Their Standard Errors by Trial Arm Adjusted for Age, Socioeconomic Score, Home Score, Recruitment Location, KABC Learning and Delayed Recall Scores at Baseline, and Outcome Score at Baseline
| CCRT LS mean (SE) | Limited CCRT LS mean (SE) | Control LS mean (SE) | p-Value for comparison by arm | p-Value for CCRT vs. control | p-Value for limited CCRT vs. control | |
|---|---|---|---|---|---|---|
| KABC-II sequential processinga | 71.56 (1.35) | 73.46 (1.18) | 71.88 (1.29) | .50 | .87 | .35 |
| KABC-II simultaneous processing | 68.74 (1.59) | 65.40 (1.38) | 64.75 (1.51) | .14 | .06 | .74 |
| KABC-II learning | 71.09 (1.41) | 72.04 (1.23) | 68.19 (1.35) | .08 | .12 | .03 |
| KABC-II knowledge** | 72.10 (1.44) | 67.66 (1.23) | 66.11 (1.37) | <.01 | <.01 | .39 |
| KABC-II planning* | 62.67 (1.05) | 61.88 (1.07) | 58.93 (1.18) | .04 | .02 | .07 |
| KABC-II delayed recall | 70.92 (1.23) | 70.79 (1.08) | 68.39 (1.18) | .20 | .12 | .12 |
| KABC-II nonverbal index | 62.26 (1.42) | 63.07 (1.25) | 58.96 (1.35) | .06 | .08 | .02 |
| KABC-II mental processing index* | 62.61 (0.87) | 62.50 (0.76) | 59.84 (0.83) | .02 | .02 | .02 |
| CogState, maze chase correct moves per second** | 0.41 (0.03) | 0.42 (0.03) | 0.26 (0.03) | <.01 | <.01 | <.01 |
| CogState, maze learning correct moves per second** | 0.20 (0.02) | 0.21 (0.02) | 0.13 (0.02) | <.01 | <.01 | <.01 |
| CogState, detection time card turned (log mean msec) | 2.87 (0.02) | 2.86 (0.02) | 2.86 (0.02) | .72 | .44 | .86 |
| CogState, identification time card red suit (log mean msec) | 3.00 (0.01) | 3.00 (0.01) | 2.99 (0.01) | .62 | .52 | .34 |
| CogState, accuracy in one-card learning* | 0.64 (0.02) | 0.60 (0.02) | 0.57 (0.02) | .03 | <.01 | .24 |
| CogState, accuracy in one-back card memory | 0.68 (0.04) | 0.63 (0.04) | 0.67 (0.04) | .65 | .90 | .47 |
Standardardized scores (using age-based norms).
p < .05 for CCRT versus limited CCRT.
p < .01 for CCRT versus limited CCRT.
p-Values in bold are statistically significant.
From first to last training session, both the full and limited CCRT arms had comparable significant improvements on all of the CLIE measures of fidelity of training. Table 4 includes the correlations between CLIE from the last training session and the KABC, CogState, TOVA, Bruininks/Oseretsky test for motor proficiency–second edition (BOT-2), BRIEF, and CBCL principal outcomes immediately following CCRT (2 months). CLIE for the last training session was highly correlated with KABC-II and CogState outcomes that significantly improved from full CCRT (p < .001). Even the TOVA outcomes, which did not significantly improve from CCRT, were significantly correlated to CLIE for both the CCRT and limited CCRT treatment arms (Table 4).
Table 4.
Associations Between Captain's Log Performance Success Composite Measure from the Last Training Session with KABC and CogState, TOVA, BOT-2, BRIEF, and CBCL Outcomes at 2 months
| Neuropsychology outcomes | Correlation, p-value, N both CCRT arms combined | Correlation, p-value, N CCRT | Correlation, p-value, N Limited CCRT |
|---|---|---|---|
| KABC-II sequential processing | 0.16890 | 0.28905 | 0.24716 |
| .0865 | .0377 | .0773 | |
| 104 | 52 | 52 | |
| KABC-II simultaneous processing | 0.21027 | 0.40636 | 0.31516 |
| .0322 | .0028 | .0229 | |
| 104 | 52 | 52 | |
| KABC-II learning | 0.37490 | 0.46468 | 0.25711 |
| <.0001 | .0005 | .0658 | |
| 104 | 52 | 52 | |
| KABC-II knowledge | 0.14579 | 0.19680 | 0.22009 |
| .1398 | .1620 | .1169 | |
| 104 | 52 | 52 | |
| KABC-II planning | 0.23646 | 0.19609 | 0.19574 |
| .0248 | .1865 | .2084 | |
| 90 | 47 | 43 | |
| KABC-II delayed recall | 0.26410 | 0.29487 | 0.18437 |
| .0067 | .0338 | .1907 | |
| 104 | 52 | 52 | |
| KABC-II nonverbal index | 0.23857 | 0.47394 | 0.18239 |
| .0147 | .0004 | .1956 | |
| 104 | 52 | 52 | |
| KABC-II mental processing index | 0.29574 | 0.43314 | 0.23794 |
| .0023 | .0013 | .0894 | |
| 104 | 52 | 52 | |
| TOVA% omission errors | −0.28890 | −0.56424 | −0.44374 |
| .0029 | <.0001 | .0010 | |
| 104 | 52 | 52 | |
| TOVA% commission errors | −0.35133 | −0.42219 | −0.47518 |
| .0003 | .0018 | .0004 | |
| 104 | 52 | 52 | |
| TOVA response time | −0.31594 | −0.53459 | −0.48492 |
| .0011 | <.0001 | .0003 | |
| 104 | 52 | 52 | |
| TOVA response time variability | −0.47159 | −0.66011 | −0.62921 |
| <.0001 | <.0001 | <.0001 | |
| 104 | 52 | 52 | |
| TOVA D prime signal detection score | 0.45219 | 0.65211 | 0.60422 |
| <.0001 | <.0001 | <.0001 | |
| 104 | 52 | 52 | |
| TOVA ADHD index | 0.25659 | 0.36138 | 0.23341 |
| .0086 | .0085 | .0959 | |
| 104 | 52 | 52 | |
| BOT-2 total motor score | 0.14691 | 0.27669 | 0.27980 |
| .1367 | .0471 | .0445 | |
| 104 | 52 | 52 | |
| CogState, maze chase correct moves per second | 0.32833 | 0.40787 | 0.46928 |
| .0007 | .0027 | .0004 | |
| 104 | 52 | 52 | |
| CogState, maze learning correct moves per second | 0.30791 | 0.48046 | 0.50062 |
| .0015 | .0003 | .0002 | |
| 104 | 52 | 52 | |
| CogState, detection time: playing card turned | −0.20304 | −0.38218 | −0.38038 |
| .0387 | .0052 | .0054 | |
| 104 | 52 | 52 | |
| CogState, identification time: red playing card turned | −0.19251 | −0.48910 | −0.33404 |
| .0503 | .0002 | .0155 | |
| 104 | 52 | 52 | |
| CogState, accuracy in one-card learning | 0.20128 | 0.29283 | 0.47647 |
| .0405 | .0351 | .0004 | |
| 104 | 52 | 52 | |
| CogState, accuracy in one-back card memory | 0.39737 | 0.61549 | 0.58064 |
| <.0001 | <.0001 | <.0001 | |
| 104 | 52 | 52 | |
| BRIEF behavior regulation index | −0.21421 | −0.12993 | −0.19023 |
| .0392 | .3841 | .2054 | |
| 93 | 47 | 46 | |
| BRIEF metacognition index | −0.33242 | −0.39218 | −0.32614 |
| .0011 | .0064 | .0270 | |
| 93 | 47 | 46 | |
| BRIEF global executive composite index | −0.28405 | −0.29307 | −0.31017 |
| .0058 | .0456 | .0359 | |
| 93 | 47 | 46 | |
| CBCL externalizing total | −0.19587 | −0.16002 | −0.15208 |
| .0463 | .2571 | .2818 | |
| 104 | 52 | 52 | |
| CBCL internalizing total | −0.14429 | 0.02626 | −0.02828 |
| .1439 | .8534 | .8423 | |
| 104 | 52 | 52 | |
| CBCL total | −0.25922 | −0.12003 | −0.22522 |
| .0079 | .3967 | .1084 | |
| 104 | 52 | 52 |
Moderate correlations of 0.40 or higher in absolute value are bolded.
Table 5 contains a summary of longitudinal models with the interaction of trial arm by HIV immunological measures. The coefficients (slopes for each HIV immunologic measure) are compared for CCRT versus control. These are presented in this Table along with their standard errors. The immunological measures of disease status at baseline (viral load, CD4 counts, CD4 activation, CD8 counts, CD8 activation) were consistently predictive of a steeper slope (greater training benefit for CCRT vs. control arm) for KABC-II Planning, CogState card detection speed (one-step attention measure), and card identification speed (two-step attention) (Table 5).
Table 5.
Summary of Longitudinal Models with Group by Biological Measure at Baseline Interaction: Slopes for Computerized Cognitive Rehabilitation Training Versus Control, Their Standard Errors, Adjusted for Age, Being on HAART at Intake, Socioeconomic Score, Home Score, Recruitment Location, KABC Learning and Delayed Recall Scores At Baseline, and Outcome Score at Baseline
| Neuropsychology outcomes | Viral load: coefficient for CCRT vs. control (SE), p-value | CD4: coefficient for CCRT vs. control (SE), p-value | CD4 activation: coefficient for CCRT vs. control (SE), p-value | CD8: coefficient for CCRT vs. control (SE), p-value | CD8 activation: coefficient for CCRT vs. control (SE), p-value |
|---|---|---|---|---|---|
| KABC-II sequential processing | 0.17 (0.24), .49 | −0.17 (0.09), .05 | 0.03 (0.27), .92 | 0.23 (0.09), .02 | −0.02 (0.12), .90 |
| KABC-II simultaneous processing | −0.24 (0.29), .42 | −0.12 (0.11), .28 | 0.09 (0.32), .77 | 0.11 (0.12), .36 | 0.13 (0.14), .36 |
| KABC-II learning | 0.27 (0.26), .30 | −0.15 (0.09), .13 | 0.24 (0.29), 0.41 | 0.16 (0.10), .13 | 0.12 (0.12), .37 |
| KABC-II knowledge | −0.50 (0.24) .04 | −0.01 (0.09), .91 | −0.37 (0.27), .17 | 0.03 (0.10), .78 | −0.27 (0.12), .02 |
| KABC-II planning | −0.41 (0.21), .06 | 0.14 (0.07), .07 | −0.40 (0.20), .06 | −0.16 (0.08), .06 | −0.28 (0.09), <.01 |
| KABC delayed recall | 0.13 (0.24), .58 | −0.11 (0.08), .21 | 0.33 (0.26), .21 | 0.12 (0.09), .21 | 0.09 (0.11), .43 |
| KABC nonverbal index | −0.21 (0.25), .39 | 0.04 (0.09), .63 | −0.02 (0.27), .93 | −0.05 (0.10), .65 | −0.14 (0.12), .24 |
| KABC mental processing index | −0.01 (0.15), .97 | −0.08 (0.05), .14 | −0.03 (0.17), .86 | 0.10 (0.06), .10 | 0.00 (0.07), .94 |
| TOVA% omission errors | −0.16 (0.53), .77 | 0.34 (0.19), .07 | −0.49 (0.58), .40 | −0.33 (0.21), .12 | −0.18 (0.25), .49 |
| TOVA% commission errors | 0.19 (.32), .55 | 0.20 (0.11), .07 | 0.01 (0.35), .98 | −0.19 (0.13), .13 | −0.06 (0.15), .69 |
| TOVA response time | 0.58 (3.25), .86 | 1.04 (1.17), .38 | −3.33 (3.51), .34 | −0.73 (1.29), .57 | −1.51 (1.52), 0.32 |
| TOVA response time variability | 0.12 (2.03), .95 | 0.86 (0.73), .24 | −2.13 (2.20), .33 | −0.63 (0.80), .43 | −0.64 (0.97), .51 |
| TOVA D prime signal detection | −0.01 (0.03), .65 | −0.02 (0.01), .07 | 0.02 (0.03), .64 | 0.03 (0.01), .12 | 0.0 (0.02), .79 |
| TOVA ADHD index | −0.04 (0.10), .70 | −0.03 (0.04), .35 | 0.11 (0.11), .32 | 0.02 (0.04), .67 | 0.01 (0.05), .87 |
| CogState, maze chase Correct moves per second | 0.04 (.004), .75 | −0.01 (0.002), .40 | 0.00 (0.01), .96 | 0.0 (0.01), .56 | 0.00 (0.01), .45 |
| CogState, maze learning correct moves per second | 0.05 (0.003), .19 | −0.001 (0.001), .36 | .000 (.003), .96 | 0.001 (0.001), .61 | 0.003 (0.002), .08 |
| CogState, detection time: playing card turning | −0.004 (.003), .15 | 0.002 (0.001), .04 | −0.006 (0.003), .05 | −0.003 (0.001), .03 | −.003 (0.001), .02 |
| CogState, identification time: red playing card turning | −0.004 (0.003), .09 | 0.002 (0.001), .05 | −0.004 (0.003), .20 | −0.002 (0.001), .05 | −.002 (0.001), .18 |
| CogState, accuracy in one-card learning | 0.003 (0.003), .88 | 0.001 (0.001), .72 | −0.005 (0.004), .23 | −0.002 (0.002), .30 | −0.003 (0.002), .19 |
| CogState, accuracy in one-back card memory | 0.06 (0.007), .48 | −0.003 (0.003), .22 | 0.006 (0.008), .45 | 0.001 (0.003), .61 | 0.005 (0.004), .19 |
p-Values in bold are statistically significant in terms of the bivariate relationship.
Discussion
The results from this study demonstrate that CCRT-based programs can be adapted for resource poor and rural non-Western settings, and children can be motivated to take part in these extended training regimens. It is also important to note that those intervention children deriving the greatest benefit from CCRT were also those who were the most clinically stable study children on HAART at enrollment. These children tended to have higher CD4, lower CD4 and CD8 activation, and be virally suppressed Our preliminary study also demonstrated the expected improvements in cognitive testing following training, with generalization to neurocognitive performance measures entirely different than the nature of the specific skills needed for Captain's Log CCRT performance. Trainers consistently observed in their session that the children seemed to like and be well-motivated to take part in these game-based training exercises. This would suggest improved motivation and learning adherence, which have been associated with increased benefit from computerized training.10 In addition, although examiners were present during training and observed that all children maintained training regimens, further research with similar CCRT programs may demonstrate that it can be easily distributed in the field for children to use on their own at home or in the school setting.
Astle et al. recently published a randomized controlled trial of CCRT emphasizing WM training (see www.cogmed.edu) with normal school children in Cambridge, UK.14 They compared a full CCRT treatment arm of children receiving from 20 to 25 sessions of Cogmed WM (visual and verbal) CCRT to children receiving a nontitrating version of Cogmed training. They used magnetoencephalography (MEG) to evaluate changes in resting-state connectivity between brain regions underpinning WM performance based on their foundational exploratory work with MEG.43 Changes in the brain connectivity regions they examined from before to after training were significantly more pronounced in the full CCRT arm compared to the limited CCRT arm, although some changes were apparent there as well. Furthermore, the degree of resting-state changes in the connectivity among these brain regions was systematically related to the degree of improvement in WM performance resulting from training in individual children. These findings led Astle and colleagues to conclude that theirs was the first systematic demonstration that CCRT in children enhances neurophysiological brain connectivity intra- and interhemispherically between brain regions known to be related to verbal and visual–spatial WM, respectively (frontoparietal networks and both lateral occipital complex and inferior temporal cortex).14,43
Because this effect is observed “at rest,” and children are not performing any task during the scan, neither could these training-related differences in brain region connectivity be attributed to differences in motivation or strategy nor could their documented brain effects be attributed to differences in blood flow or metabolism, since MEG relies on the electromagnetic field activity of the brain at the microregional level. Furthermore, improvements in WM after training were associated with increased strength of neural connectivity at rest, with the magnitude of these specific neurophysiological changes being mirrored by individual gains in untrained WM performance. These brain/behavior CCRT findings of Astle and colleagues are important in substantiating the potential neurophysiological basis of positive brain plasticity in children, hypothesized to be foundational to this performance gains in the application of CCRT with Ugandan children with HIV.
Through this and a set of other studies our group has completed using CCRT in rural settings with HIV and severe malaria survivors, we have demonstrated that the intervention is feasible and effective.10,12,13,44 This study, however, is the first to do so within a more rigorous RCT study utilizing full and limited CCRT and passive control arms. The Astle et al. study lacked a passive control arm for performance and connectivity reference. It also lacked a further posttraining follow-up to evaluate whether WM gains and the enhanced neuroconnectivity from CCRT endured months following the completion of training.14 Our findings suggest that they might.
The next step will be to evaluate ways of bringing such CCRT interventions to scale at a school- or community-wide level. For example, in a pilot intervention with passive controls from this study, we evaluated the neuropsychological benefits of a cognitive games package developed for the African context at Michigan State University, called brain powered games (BPG).45 Twenty-four training sessions of BPG over 2 months significantly improved TOVA and CogState performance in these children,45 with anecdotal evidence of how much children appreciated the BPG based on their emphasis on African motif stimuli and music. A next step might be to encourage in-country and local capacity for stakeholders to incubate and evaluate such game packages for scale-up and community-based intervention programs and initiatives. Such programs would take advantage of increased use of smartphones and tablets within the community among children and adolescents.
Conclusions
CCRT interventions can be an effective and viable means of neurocognitive rehabilitation in children with HIV in low-resource settings. The titration feature of CRRT is important for training benefit. Clinically stable children with HIV seemed to derive great neuropsychological benefit from CCRT. This suggests that the combination of HAART and cognitive rehabilitation is important for these children. Future studies are planned evaluating cognitive games for tablets and smartphones that can make cognitive evaluation and CCRT accessible on a mobile network.
Clinical Trial Registry
ClinicalTrials.gov Identifier: NCT00926003, Submitted June 22, 2009.
Acknowledgments
Financial support: This work was funded by the National Institutes of Health (M.J.B., B.G., N.N., grant number R34 MH084782); a Michigan State University (MSU) Clinical Translational Science Implementation (CTSI) (M.J.B. grant number 13-CTSI-204); an MSU College of Osteopathic Medicine (COM) Discretionary Funding Initiative (DFI) award to M.J.B. (12-DFI-COM); an MSU Department of Neurology faculty start-up funding provision to M.J.B.; a University of Michigan (U-M) Global Reach Faculty Mentored Structured Overseas Project (B.G., M.J.B.); and a U-M Global Reach Faculty Development Award (B.G., M.J.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any of the other funders.
Medical care and ARV treatment were provided by Walter Reed Hospitals clinic at Kayunga District Hospital, and their medical support of the children is greatly appreciated. Cognitive training, assessment, and on-site study management were conducted by members of the Global Health Uganda pediatric HIV study team in Kayunga, Uganda. Team members were Sylvia Malemo Elyanu, Janet Nafula Omita, Betty Nyangoma, Peter Bazira, Godfrey Nyanja Musoke, Agatha Kuteesa, Mohammed Ssemogerere, and Madina Muteesi. Michigan State University medical students who contributed as research interns for the study included Elizabeth Schut, Erin Lorenz, Christopher Adams, and Hailey Wouters. University of Michigan medical students Raina Vachani, Sarah Michael, Rashmi Patil, Andrew Gardner, and Moona Arabkhazaeli also contributed through the support of the University of Michigan Medical School global Reach summer faculty research mentoring program. Jacquelyn Moore from the Chicago Professional School of Psychology also assisted in training team members on KABC-II administration.
All of the authors have contributed substantially to this study and merit inclusion as coauthors. M.J.B. as Principal Investigator designed the study, wrote the grants that funded it, supervised all aspects of the protocol development and implementation, helped program Captain's Log for CCRT, worked on the data analyses, and wrote the article. N.N. supervised the IRB approvals in Uganda, supported logistical and personnel oversight, and contributed to the writing of the article. A.S. verified data quality assurance, completed all data analyses, developed the results Tables, and contributed to the writing of the article. R.O.O. contributed to patient selection and recruitment, clinical coordination, and treatment support for the children, helped supervise study logistics and personnel, and contributed to the writing of the article. B.G. helped design the study, write the grants that funded this project, and program Captain's Log for CCRT, helped with CogState assessment access and programming and contributed to the interpretation of the study findings, and helped write the article.
Author Disclosure Statement
None of the authors have any conflicts of interest to disclose with respect to this study.
References
- 1.Ciaranello AL, Chang Y, Margulis AV, Bernstein A, Bassett IV, Losina E, et al. : Effectiveness of pediatric antiretroviral therapy in resource-limited settings: A systematic review and meta-analysis. Clin Infect Dis 2009;49:1915–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ: Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis 2008;8:477–489 [DOI] [PubMed] [Google Scholar]
- 3.Laughton B, Cornell M, Boivin M, Van Rie A: Neurodevelopment in perinatally HIV-infected children: A concern for adolescence. J Int AIDS Soc 2013;16:18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellins CA, Malee KM: Understanding the mental health of youth living with perinatal HIV infection: Lessons learned and current challenges. J Int AIDS Soc 2013;16:18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisiacchi PS, Suppiej A, Laverda A: Neuropsychological evaluation of neurologically asymptomatic HIV-infected children. Brain Cogn 2000;43:49–52 [PubMed] [Google Scholar]
- 6.Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L: Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART). Dev Neuropsychol 2006;30:633–657 [DOI] [PubMed] [Google Scholar]
- 7.Koekkoek S, de Sonneville LM, Wolfs TF, Licht R, Geelen SP: Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol 2008;12:290–297 [DOI] [PubMed] [Google Scholar]
- 8.Tardieu M, Mayaux MJ, Seibel N, Funck-Brentano I, Straub E, Teglas JP, et al. : Cognitive assessment of school-age children infected with maternally transmitted human immunodeficiency virus type 1. J Pediatr 1995;126:375–379 [DOI] [PubMed] [Google Scholar]
- 9.Boivin MJ, Ruel TD, Boal HE, Bangirana P, Cao H, Eller LA, et al. : HIV-subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy-naive Ugandan children. AIDS 2010;24:1163–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangirana P, Boivin MJ, Giordani B: Computerized cognitive rehabilitation therapy (CCRT) for African Children: Evidence for neuropsychological benefit and future directions. In: Neuropsychology of Children in Africa: Perspectives on Risk and Resilience (Boivin MJ, Giordani B, eds.) Springer Science+Business Media, New York NY, 2013, pp. 277–298 [Google Scholar]
- 11.Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, et al. : A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology 2010;24:667–673 [DOI] [PubMed] [Google Scholar]
- 12.Bangirana P, Allebeck P, Boivin MJ, John CC, Page C, Ehnvall A, et al. : Cognition, behaviour and academic skills after cognitive rehabilitation in Ugandan children surviving severe malaria: A randomised trial. BMC Neurol 2011;11:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangirana P, Giordani B, John CC, Page C, Opoka RO, Boivin MJ: Immediate neuropsychological and behavioral benefits of computerized cognitive rehabilitation in Ugandan pediatric cerebral malaria survivors. J Dev Behav Pediatr 2009;30:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Astle DE, Barnes JJ, Baker K, Colclough GL, Woolrich MW: Cognitive training enhances intrinsic brain connectivity in childhood. J Neurosci 2015;35:6277–6283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busman RA, Oka E, Giordani B, Boivin MJ: Examining the psychosocial adjustment and school performance of Ugandan children with HIV/AIDS. In: Neuropsychology of Children in Africa: Perspectives on Risk and Resilience (Boivin MJ, Giordani B, eds.) Springer Media & Business, New York NY, 2013, pp. 117–138 [Google Scholar]
- 16.Busman RA, Page C, Oka E, Giordani B, Boivin MJ: Factors contributing to the psychosocial adjustment of Ugandan preschool children with HIV/AIDS. In: Neuropsychology of Children in Africa: Perspectives on Risk & Resilience (Boivin MJ, Giordani B, eds.) Springer Media & Business Publishing, New York NY, 2013, pp. 95–115 [Google Scholar]
- 17.Rabiner DL: A Randomized trial of two promising interventions for students with attention problems. Ed.gov Archived Information: Duke University Project Abstract, 2005 [Google Scholar]
- 18.Rabiner DL: Attention research update: Promising results from two new cognitive training studies for ADHD. In: Sharp Brains: The Brain Fitness Authority. Durham, NC, 2008 [Google Scholar]
- 19.Rabiner DL, Murray DW, Skinner AT, Malone PS: A randomized trial of two promising computer-based interventions for students with attention difficulties. J Abnorm Child Psychol 2010;38:131–142 [DOI] [PubMed] [Google Scholar]
- 20.Katabira ET, Kamya MR, Kalyesubula I, Namale A: National Antiretroviral Treatment and Care Guidelines for Adults, Adolescents, and Children. Uganda Ministry of Health, Kampala, Uganda, 2008 [Google Scholar]
- 21.Sandford JA: Captain's Log. BrainTrain, Richmond, VA, 2007 [Google Scholar]
- 22.Kaufman AS, Kaufman NL: Manual for the Kaufman Assessment Battery for Children, Second Edition. American Guidance Service Publishing/Pearson Products Inc., Circle Pines, MN, 2004 [Google Scholar]
- 23.Bangirana P, Musisi S, Allebeck P, Giordani B, John CC, Opoka OR, et al. : A preliminary examination of the construct validity of the KABC-II in Ugandan children with a history of cerebral malaria. Afr Health Sci 2009;9:188–192 [PMC free article] [PubMed] [Google Scholar]
- 24.Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, et al. : Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis 2012;54:1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falleti MG, Maruff P, Collie A, Darby DG: Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J Clin Exp Neuropsychol 2006;28:1095–1112 [DOI] [PubMed] [Google Scholar]
- 26.Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, et al. : Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol 2009;24:165–178 [DOI] [PubMed] [Google Scholar]
- 27.Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, et al. : A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology 24: 667–673, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Greenberg LM: The T.O.V.A. (Version 6.X) (Computer Program). Universal Attention Disorders, Los Alamitos, CA, 1993 [Google Scholar]
- 29.Bruininks RH, Bruininks BD: BOT2: Bruininks-Oseretsky Test of Motor Proficiency, Second Edition. Pearson Assessments, Minneapolis, MN, 2005 [Google Scholar]
- 30.Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA: A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol 1995;14:13–21 [DOI] [PubMed] [Google Scholar]
- 31.Van Rie A, Mupuala A, Dow A: Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics 2008;122:e123–e128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioia GA, Espy KA, Isquith PK: Behavior Rating Inventory of Executive Function®–Preschool Version (BRIEF®-P): Professional Manual. Psychological Assessment Resources (PAR), Lutz, FL, 2003 [Google Scholar]
- 33.Familiar I, Ruisenor-Escudero H, Giordani B, Bangirana P, Nakasujja N, Opoka R, et al. : Use of the behavior rating inventory of executive function and child behavior checklist in ugandan children with HIV or a history of severe malaria. J Dev Behav Pediatr 2015;36:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruiseñor-Escudero H, Familiar I, Nakasujja N, Bangirana P, Opoka RO, Giordani B, et al. : Immunological correlates of behavioral problems in school-aged children living with HIV in Kayunga, Uganda. Global Mental Health 2015;2:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boivin MJ, Bangirana P, Nakasujja N, Page CF, Shohet C, Givon D, et al. : A year-long caregiver training program improves cognition in preschool Ugandan children with human immunodeficiency virus. J Pediatr 2013;163:1409–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boivin MJ, Bangirana P, Nakasujja N, Page CF, Shohet C, Givon D, et al. : A year-long caregiver training program to improve neurocognition in preschool Ugandan HIV-exposed children. J Dev Behav Pediatr 2013;34:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boivin MJ, Giordani B: Neuropsychological assessment of African children: Evidence for a universal basis to cognitive ability. In: Cultural Neuroscience: Cultural Influences on Brain Function (Chiao JY, ed.) Elsevier Publications, New York NY, 2009, pp. 113–135 [DOI] [PubMed] [Google Scholar]
- 38.Caldwell BM, Bradley RH: Home Observation for Measurement of the Environment. University of Arkansas Press, Little Rock, AR, 1979 [Google Scholar]
- 39.Bradley RH, Caldwell BM, Corwyn RF: The Child Care HOME Inventories: Assessing the quality of family child care homes. Early Child Res Q 2003;18:294–309 [Google Scholar]
- 40.Bangirana P, John CC, Idro R, Opoka RO, Byarugaba J, Jurek AM, et al. : Socioeconomic predictors of cognition in Ugandan children: Implications for community based interventions. PLoS One 2009;4:e7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barry SM, Johnson MA, Janossy G: Increased proportions of activated and proliferating memory CD8+ T lymphocytes in both blood and lung are associated with blood HIV viral load. J Acquir Immune Defic Syndr 2003;34:351–357 [DOI] [PubMed] [Google Scholar]
- 42.Jaeggi T, Moretti D, Kvalsvig J, Holding PA, Tjalsma H, Kortman GA, et al. : Iron status and systemic inflammation, but not gut inflammation, strongly predict gender-specific concentrations of serum hepcidin in infants in rural Kenya. PLoS One 2013;8:e57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes JJ, Woolrich MW, Baker K, Colclough GL, Astle DE: Electrophysiological measures of resting state functional connectivity and their relationship with working memory capacity in childhood. Dev Sci 2016;19:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boivin MJ, Bangirana P, Tomac R, Parikh S, Opoka RO, Nakasujja N, et al. : Neuropsychological benefits of computerized cognitive rehabilitation training in Ugandan children surviving cerebral malaria and children with HIV. BMC Proc 2008;2:P7 [Google Scholar]
- 45.Giordani B, Novak B, Sikorskii A, Bangirana P, Nakasujja N, Winn BM, et al. : Designing and evaluating brain powered games for cognitive training and rehabilitation in at-risk African children. Global Mental Health 2015;2:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]