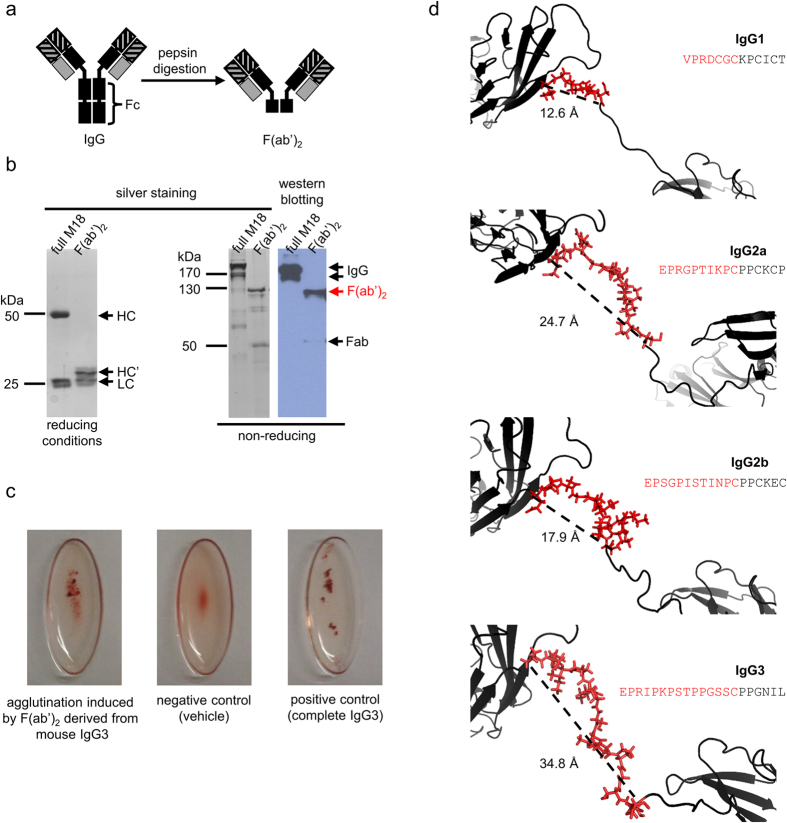
Figure 3. Structural determinants of mouse IgG3 agglutination capacity.
(a–c) RBCs agglutination induced by F(ab′)2 of M18 antibody. (a) Pepsin digestion removes the Fc fragment from the IgG molecule, resulting in F(ab′)2. (b) Purity of M18 and its F(ab′)2. An intact IgG antibody under reducing conditions migrates as two bands corresponding to heavy chain (HC, approximately 50 kDa) and light chain (LC, 25 kDa). HC’ is a heavy chain digested with pepsin. F(ab′)2 is a protein with molecular mass of approximately 120 kDa, which was confirmed by the SDS-PAGE and western blotting under non-reducing conditions. Samples in western blotting were probed with anti-mouse-Ig antibody. IgG under non-reducing conditions migrates as two or three bands. A small amount of Fab is inevitably generated during pepsin digestion. (c) Haemagglutination of group B erythrocytes induced by mouse IgG3-derived F(ab′)2, vehicle buffer, and intact parental M18 antibody. (b,c) are representative results of five independent experiments. (d) Molecular modelling of the hinge region in M18 isotype variants. For each isotype a distance between alpha carbon of the first hinge amino acid and alpha carbon of the first cysteine residue involved in the inter-heavy chain disulphide bond is indicated. The sequence of the upper hinge region is presented for each subtype in red. For the sake of clarity, only one heavy chain was presented. The models of M18 IgG1, IgG2a and IgG3 variants were generated with C-score greater than 0.9, which indicates high confidence of the predicted models. C-score calculated for the M18 IgG2b model was slightly lower and reached 0.5 due to a lack of a complete mouse IgG2b structure in PDB database that could be used as a template in the modelling algorithm.