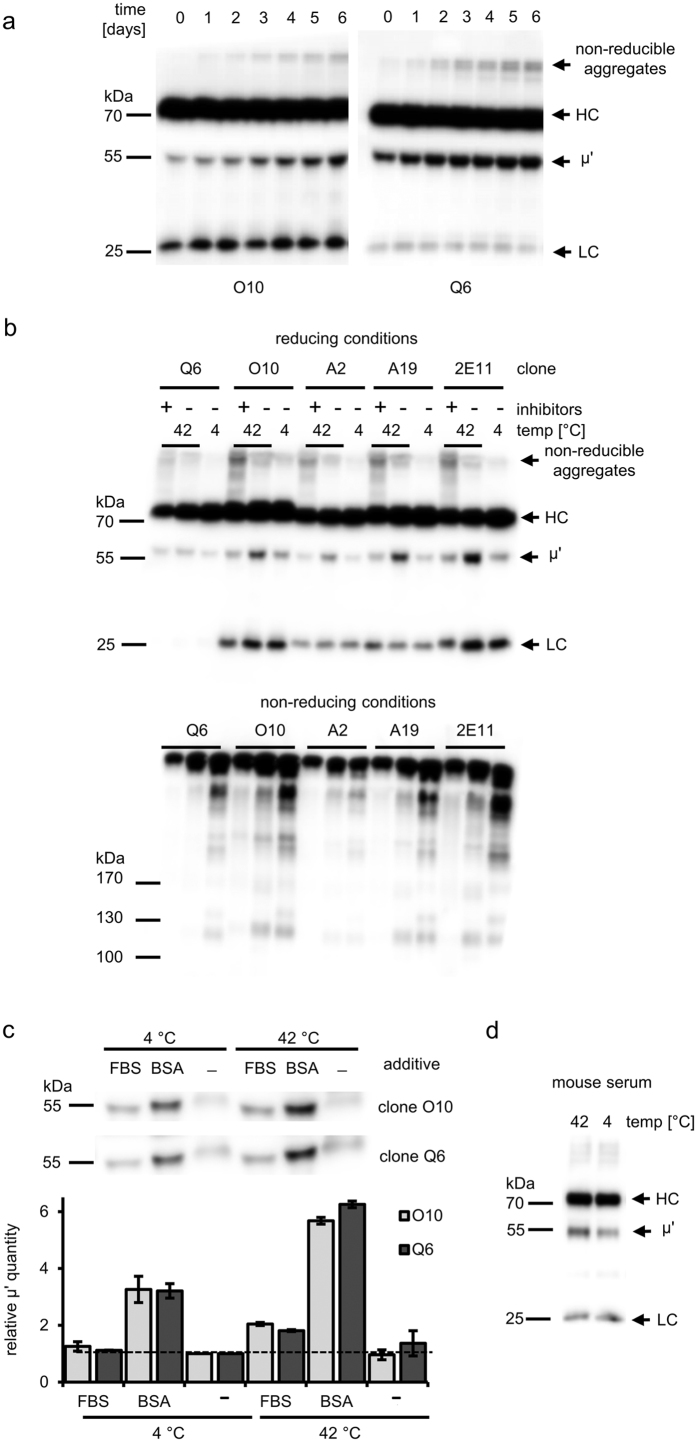
Figure 4. Chemical instability of IgM – western blotting analyses.
All samples were probed with HRP-labelled anti-mouse IgM κ polyclonal antibodies. (a) O10- and Q6-based reagents were incubated at 42 °C for 6 days. Amount of truncated heavy chain (μ′) increased in subsequent samples taken daily. Images are representative of four independent experiments. (b) Protease inhibitor cocktail prevented trimming of a heavy chain, but promoted the formation of both reducible and non-reducible disulphide-based aggregates. Amount of IgM aggregates with the high molecular mass increased after exposure to elevated temperature. Representative results of four independent experiments are shown. Blots in (b) present the same samples resolved under reducing and non-reducing conditions. HC – heavy chain, LC – light chain. (c) Protease(s) present in serum trim(s) the IgM heavy chain. Purified IgM antibodies were incubated with 5% FBS or ht-BSA (1.5 mg/ml) for 4 days at indicated temperatures. The bar chart presents relative amounts of truncated heavy chain quantified using densitometry. The intensity of a band corresponding to the μ′ chain in the control sample without additive incubated at 4 °C was defined as 1. The μ′ chain in samples with additives migrates slightly faster than in control, probably due to the presence of the large band of serum albumin, which molecular mass is approximately 66 kDa. The chart presents mean values derived from two separate blots prepared from the same samples in one experiment. Results in (c) are representative of two independent experiments. (d) Truncation of μ chain occurs in mouse serum. Samples of mouse serum were incubated in parallel at 4 and 42 °C for 4 days. The figure presents a result obtained for one serum sample representative of three out of four analysed samples collected from different mice (in one sample the levels of μ′ were similar at 4 °C and 42 °C).