Abstract
Background:
Implantation failure is one of the most important factors limiting success in IVF treatment. The majority of trials have demonstrated favorable effect of endometrial injury on implantation success rate especially in women with recurrent implantation failure, while some studies failed to detect any benefit.
Objective:
The purpose of our trial was to explore whether endometrial injury in luteal phase prior to frozen-thawed embryo transfer cycles would improve pregnancy outcomes?
Materials and Methods:
We conducted a prospective controlled trial of 93 consecutive subjects at a research and clinical center for infertility. All women were undergone frozen-thawed embryo transfer (FTE) cycles. Women in the experimental group underwent endometrial biopsy with a Pipelle catheter in luteal phase proceeding FET cycle. Primary outcomes were implantation and clinical pregnancy rates and secondary outcomes were chemical, ongoing and multiple pregnancy and miscarriage rates.
Results:
45 subjects who underwent endometrial injury (EI) were compared with 48 control group which did not include any uterine manipulation. There were no significant differences in baseline and cycle characteristics between two groups. The difference in implantation rate was trend to statistically significance, 11.8% in EI group vs. 20.5% in control group (p=0.091). The chemical, clinical and ongoing pregnancy rates were lower in EI group compared with control group but not statistically significant. The multiple pregnancy rate and miscarriage rate also were lower in EI group compared with control group.
Conclusion:
Based on results of this study, local injury to endometrium in luteal phase prior to FET cycle had a negative impact on implantation and clinical pregnancy rates.
Key Words: Endometrial injury, Frozen-thawed embryo transfer, Pipelle catheter, Implantation rate, Pregnancy rate
Introduction
Implantation failure is one of the most important factors limiting success in IVF treatment (1). Embryo implantation is a critical process of embryonic attachment to endometrium and subsequent invasion into uterine wall (2). Uterus is receptive during mid-secretory phase (days 19-23) of menstrual cycle, which is known as window of implantation (2). Implantation of embryo is a multiple process including several cytokines and growth factors, along with a dialogue between embryo and uterine endometrium (3). Numerous factors have been contributed increasing embryo implantation success (4). Majority of trials have demonstrated favorable effect of endometrial injury on implantation success rate, especially in women with recurrent implantation failure (RIF), while some studies failed to detect any benefit (5-13).
Kalma et al suggested that “local injury to endometrium causes significant changes in pattern of expression of genes related to implantation” (14). Gnainsky et al reported that “endometrial injury induces an inflammatory reaction which favors implantation” (15). Dendritic cells, natural killer cells and macrophages are employed to local injury and increased amounts of cytokines, chemokines and growth factors are secreted, thus resulting in successful implantation (15, 16).
To our knowledge, there has not been enough research due to the effectiveness of endometrial injury prior to frozen-thawed embryo transfer (FET) cycle. The purpose of our trial was to explore whether endometrial injury in luteal phase prior to FET cycle would improve pregnancy outcomes?
Materials and methods
Study design and participants
This randomized clinical trial conducted at Research and Clinical Center for Infertility, Yazd, Iran, between March 2015 to January 2016. Ethical confirmation was received from Ethic Committee of Research and Clinical Center for Infertility and written informed consent was obtained from all participants. For study population a computer-generated randomization table was created.
The inclusion criteria include: women indicated for FET treatment, had one or more frozen embryo(s) and had a normal uterine cavity (confirmed by vaginal ultrasonography). The exclusion criteria were women <40 yrs, history of endocrine disorders (hypothyroidism, diabetes mellitus), intrauterine abnormality (uterine polyp, sub-mucosal fibroma, intrauterine adhesion) and severe endometriosis diagnosed by laparoscopy or endometrioma in ultrasound scanning.
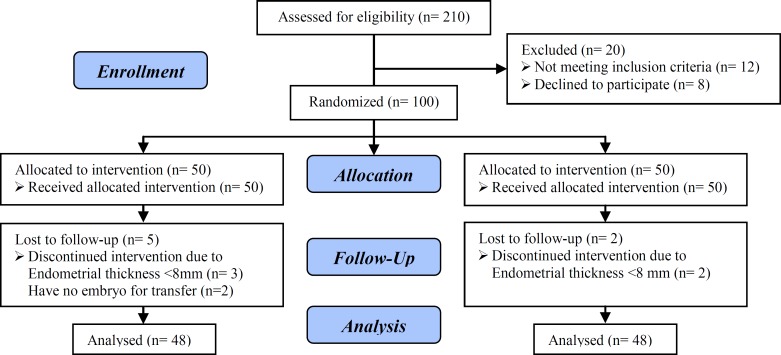
This study included initially 120 eligible participants. 20 patients excluded because of not meeting inclusion criteria (n=12) and declining to participate in the study (n=8). We allocated the remaining 100 participants in two groups: endometrial injury (EI) group (n=50) and non-endometrial injury (nEI) group (n=50). Five patients in EI group were excluded because of endometrial thickness <8 mm (n=3) and having no embryos for transfer (n=2). Two patients in nEI group were excluded because of endometrial thickness <8 mm. Finally 45 women in EI group and 48 women in nEI group were analyzed (Figure 1).
Figure 1.
Consort 2010 flow diagram of the study.
In the EI group, women underwent endometrial injury between day 21 and 23 of menstrual cycle proceeding FET cycle. EI was performed in standard fashion using Pipelle catheter (Endobiops, Prince Medical France). Catheter was introduced through the cervix up to uterine fundus. The piston was drawn back to create a negative pressure. Sheath was rotated and moved back and forth 2-3 times before it was withdrawn. In the subsequent cycle, all of women underwent our standard endometrial preparation protocol for FET cycles with estradiol valerate 6 mg daily from day 2 of the cycle.
A transvaginal ultrasound was then performed in day 13 of cycle and if endometrial thickness was ≥8 mm with a triple-line appearance, subject was started on vaginal progesterone pessary 800 mg daily (Actavis, UK) and embryo transfer was performed 3 days later with 6-8 cell frozen-thawed embryos with COOK catheter (USA) by an expert infertility fellowship.
Outcome measures
The primary outcomes were implantation and clinical pregnancy rates and secondary outcomes were chemical, ongoing, and multiple pregnancy and miscarriage rates. Chemical pregnancy rate was defined as positive hCG test 14 days after embryo transfer. Implantation rate was the sacs number seen on transvaginal ultrasound scan divided by the number of transferred embryos.
Clinical pregnancy rate was defined by ultrasound detection of gestational sac and fetal heart activity approximately 5 wks after embryo transfer. Ongoing pregnancy rate was defined as presence of fetal heart activity on ultrasound beyond 12 wks. Multiple pregnancy rates were defined as the number of multiple pregnancies divided by total number of clinical pregnancies. Miscarriage rate was defined as miscarriages number before 20 wks divided by the number of women with a positive pregnancy test.
Sample size calculation
A power analysis based on Barash et al with 30% difference in clinical pregnancy rate, demonstrated that we would require 49 patients per group to give a test with the significance of 5% and a power of 80% in this prospective randomized design (5).
Statistical analysis
SPSS software (Statistical Package for the Social Sciences version 20.0, SPSS Inc., Chicago, IL, USA) was used for all statistical calculations. Student’s t-test was used for comparing quantitative variables and 2 test used to compare categorical data. p<0.05 was considered statistically significant.
Results
In total, 93 women who underwent FET treatment were analyzed. Women were divided into two groups: EI (n=45) and nEI group which did not include any uterine manipulation in preceding luteal phase (n=48). Baseline characteristics between two groups were compared (Table I). There were no significant differences in baseline characteristics analyzed including age, type of infertility, duration and causes of infertility and number of previous embryo transfer(s) (Table II). There were no significant differences between two groups including treatment duration, endometrial thickness at progesterone initiation day, number and quality of frozen-thawed embryos transferred.
Table I.
Baseline characteristics of patients in both groups
| EI group (n=45) | non-EI group (n=48) | p-value | ||
|---|---|---|---|---|
| Age (years)* | 32.35 ± 5.61 | 31.4 ± 4.43 | 0.251$ | |
| Duration of infertility (years) * | 6.42 ± 3.62 | 6.33 ± 3.62 | 0.907 $ | |
| Type of infertility ** | ||||
| Primary | 33 (73.3%) | 39 (81.2%) | 0.459# | |
| Secondary | 12 (26.7%) | 9 (18.8%) | ||
| Causes of infertility** | ||||
| Male factor | 23 (51.1%) | 26 (54.2%) | 0.842# | |
| PCO | 8 (17.8%) | 11 (22.9%) | ||
| POF | 5 (11.1%) | 3 (6.2%) | ||
| Tubal factor | 2 (4.4%) | 4 (8.3%) | ||
| Endometriosis | 1 (2.2%) | 1 (2.1%) | ||
| Unexplained | 2 (4.4%) | 1 (2.1%) | ||
| Mixed | 4 (8.9%) | 2 (4.2%) | ||
| Number of previous transfer(s) ** | ||||
| 0 | 2 (4.4%) | 8 (16.7%) | 0.163# | |
| 1-2 | 35 (77.8%) | 33 (68.8%) | ||
| 3 | 8 (17.8%) | 7 (14.6%) | ||
Data are presented as mean±S.D.
Data presented as n (%).
Student t-test
Chi-square test
Table II.
Cycle characteristics of patients in both groups
| EI group (n=45) | non-EI group (n=48) | p-value | ||
|---|---|---|---|---|
| Treatment duration (days)* | 17.48 ± 2.58 | 17.12 ± 2.94 | 0.529$ | |
| Endometrial thickness at progesterone initiation day (mm) * | 9.13 ± 1.42 | 8.60 ± 1.37 | 0.072 $ | |
| Number of transferred embryos * | 2.11 ± 0.64 | 2.16 ± 0.63 | 0.676 $ | |
| Quality of transferred embryos n (%) | ||||
| A | 5 (11.1%) | 10 (20.8%) | 0.194# | |
| B | 35 (77.8%) | 29 (60.4%) | ||
| C | 5 (11.1%) | 9 (18.8%) | ||
Data are presented as mean±S.D.
Student t-test
Chi-square test
Pregnancy outcomes of patients in both groups are shown in table III. Implantation rate was lower in EI (11.8%) compared with nEI group (20.5%), observed difference was trend to statistically significance (p=0.091). Although chemical (26.7% vs. 39.6%), clinical (22.2% vs. 33.3%) and ongoing (22.2% vs. 31.2%) pregnancy rates were lower in EI compared with nEI group, the observed differences were short of reaching statistically significance. Multiple pregnancy (10% vs. 25%) and miscarriage rates (16.7% vs. 21.1%) were lower in EI compared with nEI group with no statistically difference.
Table III.
Pregnancy outcomes of patients in both groups
| EI group (n=45) | non-EI group (n=48) | p-value | |
|---|---|---|---|
| Implantation rate* | 11.8% ± 20.6% | 20.5% ± 27.3% | 0.091 |
| Chemical pregnancy rate ** | 12 (26.7%) | 19 (39.6%) | 0.187 |
| Clinical pregnancy rate ** | 10 (22.2%) | 16 (33.3%) | 0.233 |
| Ongoing pregnancy rate ** | 10 (22.2%) | 15 (31.2%) | 0.326 |
| Multiple pregnancy rate ** | 1 (10%) | 4 (25%) | 0.429 |
| Miscarriage rate ** | 2 (16.7%) | 4 (21.1%) | 0.763 |
Data presented as mean±S.D.
Data are presented as n (%).
Discussion
In the current study, endometrial injury performed in luteal phase preceding a FET cycle, had a negative impact on implantation and pregnancy outcomes. Previous studies have reported an improvement in clinical pregnancy and/or live birth rates after endometrial injury (1-4). The reported significant benefits in patients with RIF have made it tempting intervention to be offered to all patients prior to their IVF treatments. However, most of studies have been underpowered and there has been very limited data exploring the role of endometrial injury in FET treatment.
The role of endometrial injury in IVF was controversial. Barash et al first demonstrated that EI during the cycle preceding IVF doubled the implantation rates , clinical pregnancy, and live birth rates in women with RIF (5). Several studies confirmed the positive effect of EI on embryo implantation and clinical pregnancies at different time and with different frequencies, however, conflicting results were reported (1, 6, 7). Yeung et al demonstrated that EI performed in luteal phase of preceding cycle does not improve the ongoing pregnancy rate in unselected subfertile women undergoing IVF (8, 9).
Therefore, population, timing, technique and frequencies of endometrial injury were variable and led to different outcomes. The mechanism underlying EI action, remains unclear. Another study demonstrated that the implantation success was secondary to the development of an inflammatory reaction induced by trauma (10). It has been supposed that the injury to endometrium induces secretion of cytokines and growth factors that will stay in basal layer of endometrium for a few cycles and enhance decidualization and facilitate implantation (11-15). It has also been demonstrated that endometrial injury up-regulates the gene expression related to endometrial receptivity which optimizes endometrial development (16-18).
To our knowledge, no study has demonstrated the effectiveness of endometrial injury prior to transferring frozen-thawed embryos. The results of this study suggest that endometrial response to injury during a FET cycle is different, or does not confer the same benefit, as it does during IVF-ET cycle. An explanation for this diversity might be sought in various hypotheses about why endometrial injury is helpful for implantation which mentioned above. An alternate explanation was offered by Zhou et al called “backwards development theory”. They speculated that controlled ovarian hyperstimulation (COH) negatively affects embryo implantation through histological progression and functional changes such as pinopode maturation advancement and steroid receptor down-regulation.
The trauma to endometrium stimulates a wound repair process which creates a lag and serves to better sync the uterus with implanting embryo (19). If the “backwards development theory” explains why patients who have recently undergone COH can benefit from EI, then our results would be expected in FET cycles. It is possible that the frequency and endometrial injures timing , as well as the degree of injury, may have an impact on implantation and pregnancy outcomes. There is no consensus on optimal frequency and timing of procedure(s) required for endometrial injury to induce its maximal effect. Methodological and recruitment differences complicate the results comparison in FET cycles to those previously published for IVF-ET. Original publication by Barash et al included 4 biopsies, while other studies have been limited to 1 or 2 (2, 19).
A detrimental effect has been demonstrated when the endometrial injury was performed in transfer cycle on the oocyte retrieval day (8). In the current study, we performed a single endometrial biopsy in mid-luteal phase prior to FET cycle. This is presumed ‘window of implantation’ with the highest abundance of cytokines and growth factors in endometrium, where the endometrial injury effect, if any, may be maximized (20).
Although the recent systematic reviews and meta-analyses have concluded a beneficial effect of endometrial injury in patients with RIF, they have included non-randomized studies and only a limited number of available randomized trials were included (21, 22). When we review the available RCTs assessing the endometrial injury effect on pregnancy outcomes, most of them either did not have priori sample size calculation or well-defined primary outcome, or they were terminated before completion of recruitment (1-3, 23, 24).
These factors would have ability limited to draw reliable conclusions with adequate power. One of the limitations of current study was the absence of placebo and both our physicians and patients were not blinded to randomization. However, due to intervention nature , the physicians could not be blinded and patients would likely be aware of intervention.
Conclusion
In summary we concluded that EI in luteal phase prior to FET cycle did not improve implantation nor did it improve clinical pregnancy rates. Indeed we found that EI in luteal phase prior to FET cycle had a negative impact on implantation and pregnancy outcomes.
Currently, there is lack of good evidence to support routine endometrial injury prior to FET treatment. The lower multiple pregnancy and miscarriage rates in EI group would be a benefit effect of endometrial injury in FET treatment. The large randomized controlled trial of FET cycles might need to define the mechanism by which EI is helpful for IVF-ET cycles and if this can be applied to other treatments for infertility.
Acknowledgments
The financial supporter was Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Note
Registration ID in IRCT: IRCT2015101324512N1
Conflict of interest
The authors have no conflicts of interest related to the subject matter or materials discussed in this article.
References
- 1.Karimzadeh MA, Ayazi Rozbahani M, Tabibnejad N. Endometrial local injury improves the pregnancy rate among recurrent implantation failure patients undergoing in vitro fertilisation/intra cytoplasmic sperm injection: a randomised clinical trial. Aust N Z J Obstet Gynaecol. 2009;49:677–680. doi: 10.1111/j.1479-828X.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 2.Raziel A, Schachter M, Strassburger D, Bern O, Ron-El R, Friedler S. Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure. FertilSteril. 2007;87:198–201. doi: 10.1016/j.fertnstert.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 3.Gibreel A, Badawy A, ElfRefai W, ElfAdawi N. Endometrial scratching to improve pregnancy rate in couples with unexplained subfertility: a randomized controlled trial. J Obstet Gynaecol Res. 2013;39:680–684. doi: 10.1111/j.1447-0756.2012.02016.x. [DOI] [PubMed] [Google Scholar]
- 4.Shohayeb A, El-Khayat W. Does a single endometrial biopsy regimen (S-EBR) improve ICSI outcome in patients with repeated implantation failure? A randomised controlled trial. Eur J Obstet Gynecol Reprod Biol. 2012;164:176–179. doi: 10.1016/j.ejogrb.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril. 2003;79:1317–1322. doi: 10.1016/s0015-0282(03)00345-5. [DOI] [PubMed] [Google Scholar]
- 6.Narvekar SA, Gupta N, Shetty N, Kottur A, Srinivas M, Rao KA. Does local endometrial injury in the nontransfer cycle improve the IVF-ET outcome in the subsequent cycle in patients with previous unsuccessful IVF? A randomized controlled pilot study. J Hum Reprod Sci. 2010;3:15–19. doi: 10.4103/0974-1208.63116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiboni GM, Giampietro F, Gabriele E, Di Donato V, Impicciatore GG. Impact of a single endometrial injury on assisted reproductive technology outcome: a preliminary observational study. J Reprod Med. 2011;56:504–506. [PubMed] [Google Scholar]
- 8.Karimzade MA, Oskouian H, Ahmadi S, Oskouian L. Local injury to the endometrium on the day of oocyte retrieval has a negative impact on implantation in assisted reproductive cycles: a randomized controlled trial. Arch Gynecol Obstet. 2010;281:499–503. doi: 10.1007/s00404-009-1166-1. [DOI] [PubMed] [Google Scholar]
- 9.Yeung TW, Chai J, Li RH, Lee VC, Ho PC, Ng EH. The effect of endometrial injury on ongoing pregnancy rate in unselected subfertile women undergoing in vitro fertilization: a randomized controlled trial. Hum Reprod. 2014;29:2474–2481. doi: 10.1093/humrep/deu213. [DOI] [PubMed] [Google Scholar]
- 10.Dekel N, Gnainsky Y, Granot I, Mor G. Review article: Inflammation and implantation. Am J Reprod Immunol. 2010;63:17–21. doi: 10.1111/j.1600-0897.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn C, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972;7:82–86. doi: 10.1093/biolreprod/7.1.82. [DOI] [PubMed] [Google Scholar]
- 12.Sharkey A. Cytokines and implantation. Rev Reprod. 1998;3:52–61. doi: 10.1530/ror.0.0030052. [DOI] [PubMed] [Google Scholar]
- 13.Akita S, Ishihara H, Abdur RM, Fujii T. Leukemia inhibitory factor gene improves skin allograft survival in the mouse model1. Transplantation. 2000;70:1026–1031. doi: 10.1097/00007890-200010150-00007. [DOI] [PubMed] [Google Scholar]
- 14.Basak S, Dubanchet S, Zourbas S, Chaouat G, Das C. Expression of Pro-inflammatory Cytokines in Mouse Blastocysts During Implantation: Modulation by Steroid Hormones. Am J Reprod Immunol. 2002;47:2–11. doi: 10.1034/j.1600-0897.2002.1o047.x. [DOI] [PubMed] [Google Scholar]
- 15.Schlitzer A, McGovern N, Ginhoux F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin Cell Dev Biol. 2015;41:9–22. doi: 10.1016/j.semcdb.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Kalma Y, Granot I, Gnainsky Y, Or Y, Czernobilsky B, Dekel N, et al. Endometrial biopsy-induced gene modulation: first evidence for the expression of bladder-transmembranal uroplakin Ib in human endometrium. Fertil Steril. 2009;91:1042–1049. doi: 10.1016/j.fertnstert.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Almog B, Shalom-Paz E, Dufort D, Tulandi T. Promoting implantation by local injury to the endometrium. Fertil Steril. 2010;94:2026–2029. doi: 10.1016/j.fertnstert.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 18.Gnainsky Y, Granot I, Aldo P, Barash A, Or Y, Mor G, et al. Biopsy-induced inflammatory conditions improve endometrial receptivity: the mechanism of action. Reproduction. 2015;149:75–85. doi: 10.1530/REP-14-0395. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Li R, Wang R, Huang HX, Zhong K. Local injury to the endometrium in controlled ovarian hyperstimulation cycles improves implantation rates. Fertil Steril. 2008;89:1166–1176. doi: 10.1016/j.fertnstert.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 20.Gnainsky Y, Granot I, Aldo PB, Barash A, Or Y, Schechtman E, et al. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil Steril. 2010;94:2030–2036. doi: 10.1016/j.fertnstert.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Toukhy T, Sunkara S, Khalaf Y. Local endometrial injury and IVF outcome: a systematic review and meta-analysis. Reprod Biomed Online. 2012;25:345–354. doi: 10.1016/j.rbmo.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Potdar N, Gelbaya T, Nardo LG. Endometrial injury to overcome recurrent embryo implantation failure: a systematic review and meta-analysis. Reprod Biomed Online. 2012;25:561–571. doi: 10.1016/j.rbmo.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Baum M, Yerushalmi GM, Maman E, Kedem A, Machtinger R, Hourvitz A, et al. Does local injury to the endometrium before IVF cycle really affect treatment outcome? Results of a randomized placebo controlled trial. Gynecol Endocrinol. 2012;28:933–936. doi: 10.3109/09513590.2011.650750. [DOI] [PubMed] [Google Scholar]
- 24.Nastri C, Ferriani R, Raine‐Fenning N, Martins W. Endometrial scratching performed in the non-transfer cycle and outcome of assisted reproduction: a randomized controlled trial. Ultrasound ObstetGynecol. 2013;42:375–382. doi: 10.1002/uog.12539. [DOI] [PubMed] [Google Scholar]