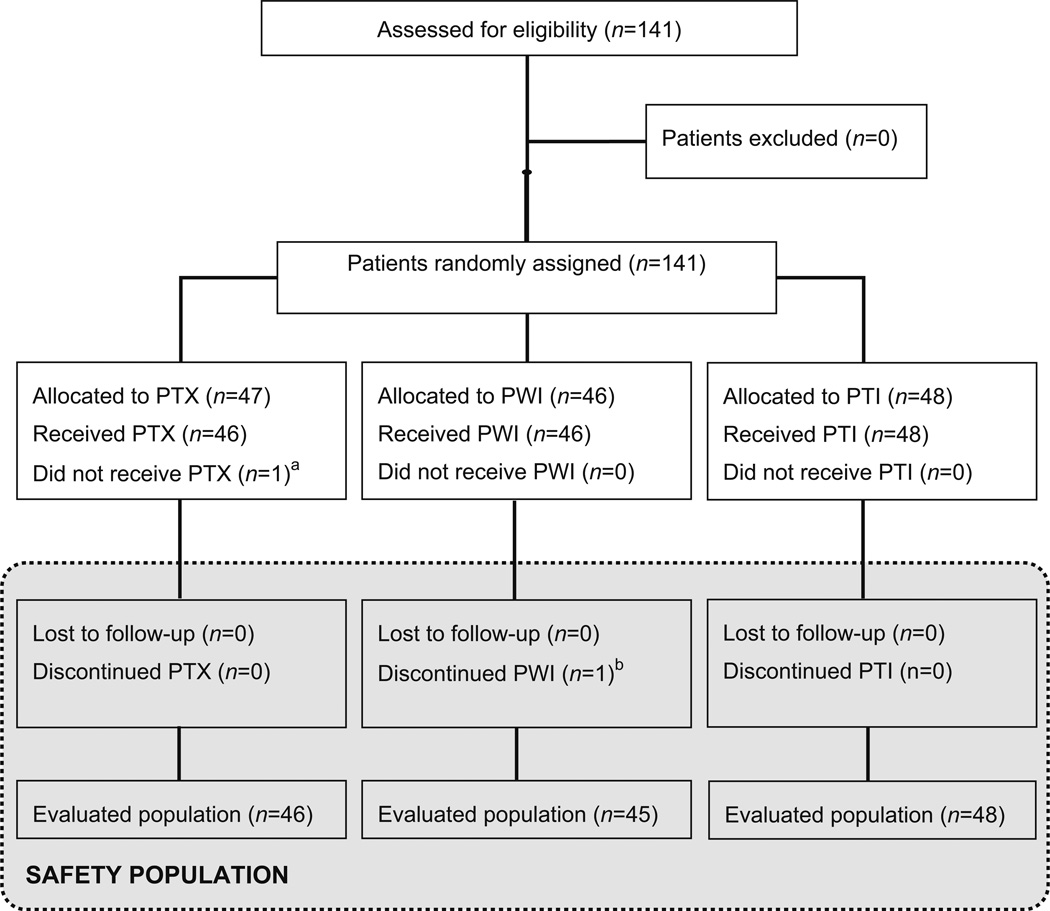
Fig. 1.
Patient disposition (CONSORT diagram). 141 patients with Stage II–IIIA TNBC were randomly assigned to receive PTX (80 mg/m2, d 1; n = 47) alone or in combination with iniparib, on either a once-weekly (PWI) (11.2 mg/kg, d1; n = 46) or twice-weekly (PTI) (5.6 mg/kg, d1, 4; n = 48) schedule for 12 weeks. a Patient was not treated due to investigator’s decision. b Patient discontinued treatment at cycle 3 due to a contralateral breast cancer present at baseline discovered retrospectively