Fig. 3.
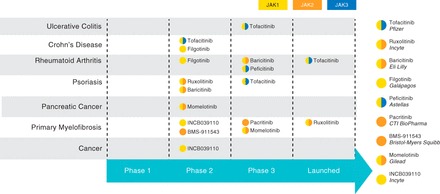
JAK inhibitors, showing all JAK inhibitor profiles, their current stage of development, and their reported JAK selectivity. Currently, tofacitinib (a JAK1/JAK3 inhibitor) and filgotinib (a JAK1 inhibitor) are the only agents undergoing clinical testing for IBD indications. Other JAK inhibitors in advanced stages of clinical development for the treatment of cytokine-mediated diseases include JAK1/JAK2 inhibitors baricitinib, momelotinib, and ruxolitinib (the latter of which is approved for the treatment of myelofibrosis), JAK1/JAK3 inhibitor peficitinib, and JAK2 inhibitor pacritinib [Source: Thomson Reuters Cortellis (74)]. Reported selectivity is indicated for each JAK inhibitor.
