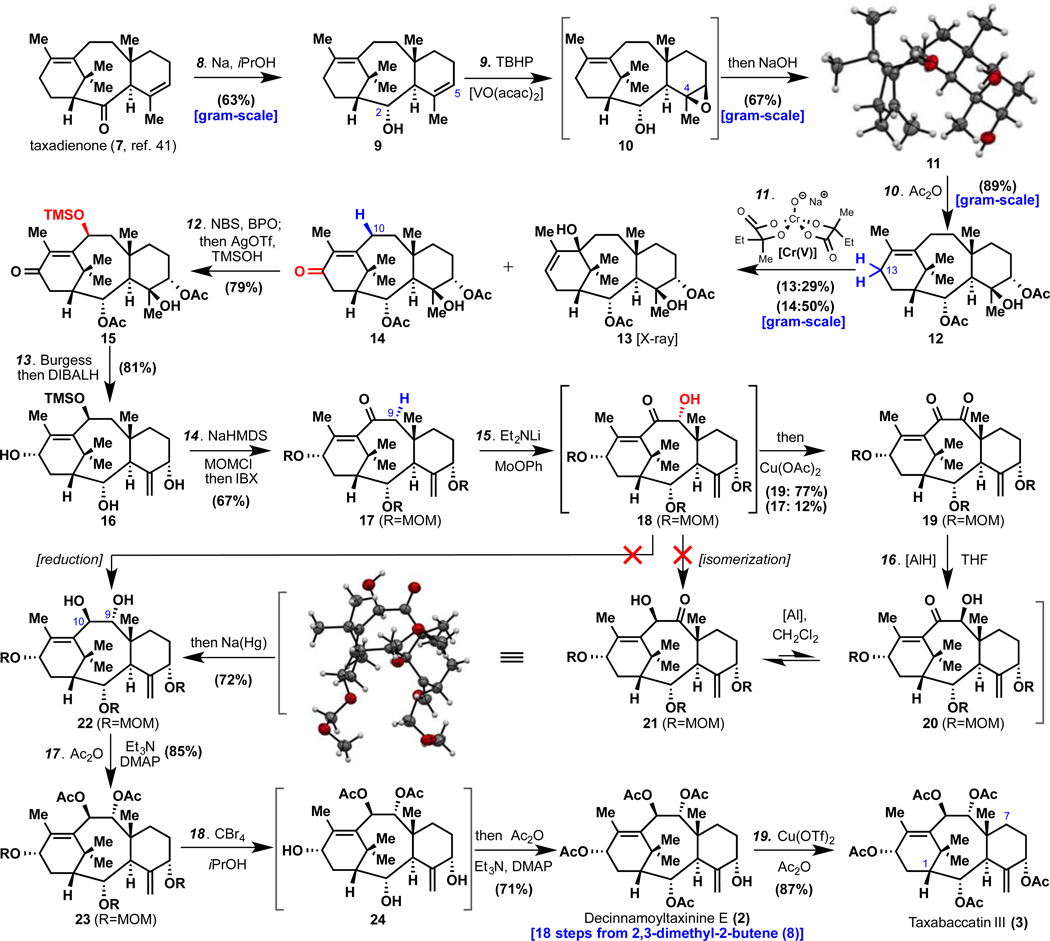
Figure 2.
Conditions: 8. Na (5 × 7 equiv., each portion added every 30 min), iPrOH, 80 °C, 3 h, 63% (11% epi-9, see SI); 9. VO(acac)2 (0.05 equiv.), TBHP (1.1 equiv.), DCE, 0→23 °C, 2 h; then aq. NaOH (3M, 8.3 equiv.), DMSO, 140 °C (sealed tube), 4 h, 67%; 10. Ac2O (6 equiv.), Et3N (8 equiv.), DMAP (0.2 equiv.), THF, 60 °C, 10 h, 89%; 11. Cr(V) (4 equiv.), 15-crown-5 (5 equiv.), MnO2 (20 equiv.), tBuOMe, 90 °C (sealed tube), 36 h, 14 50%, 13 29%; 12. NBS (1.05 equiv.), BPO (0.2 equiv.), CCl4, 80 °C, 15 min; then TMSOH (xs.), 2,6-tert-butylpyridine (5 equiv.), AgOTf (5 equiv.), toluene, 23 °C, 1.5 h, 79%; 13. Burgess reagent (2 equiv.), toluene, 80 °C, 1 h; then DIBALH (15 equiv.), toluene, −78→23 °C, 15 min, 81%; 14. KHMDS (12 equiv.), MOMCl (12 equiv.), THF, −78→23 °C, 30 min; then IBX (26 equiv.), DMSO, 80 °C, 20 h, 67%; 15. Et2NLi (2 equiv.), MoOPh (4 equiv.), THF, 23→−20→23 °C, 20 min; then Cu(OAc)2 (40 equiv.), MeOH, 23 °C, 5 h, 19 77%, 17 12%; 16. LiAlH(OtBu)sBu2 (2.5 equiv.), THF, 23 °C, 1 min; then H2O (66 equiv.), evaporated to dryness and CH2Cl2, 23 °C, 8 h; Na (Hg) (2.2 equiv.), MeOH, 23 °C, 30 min, 72%; 17. Ac2O (10 equiv.), Et3N (10 equiv.), DMAP (1 equiv.), THF, 60 °C, 9 h, 85%; 18. CBr4 (50 equiv.), iPrOH, 82 °C, 8 min; then Ac2O (360 equiv.), Et3N (96 equiv.), DMAP (100 equiv.), 23 °C, 30 min.