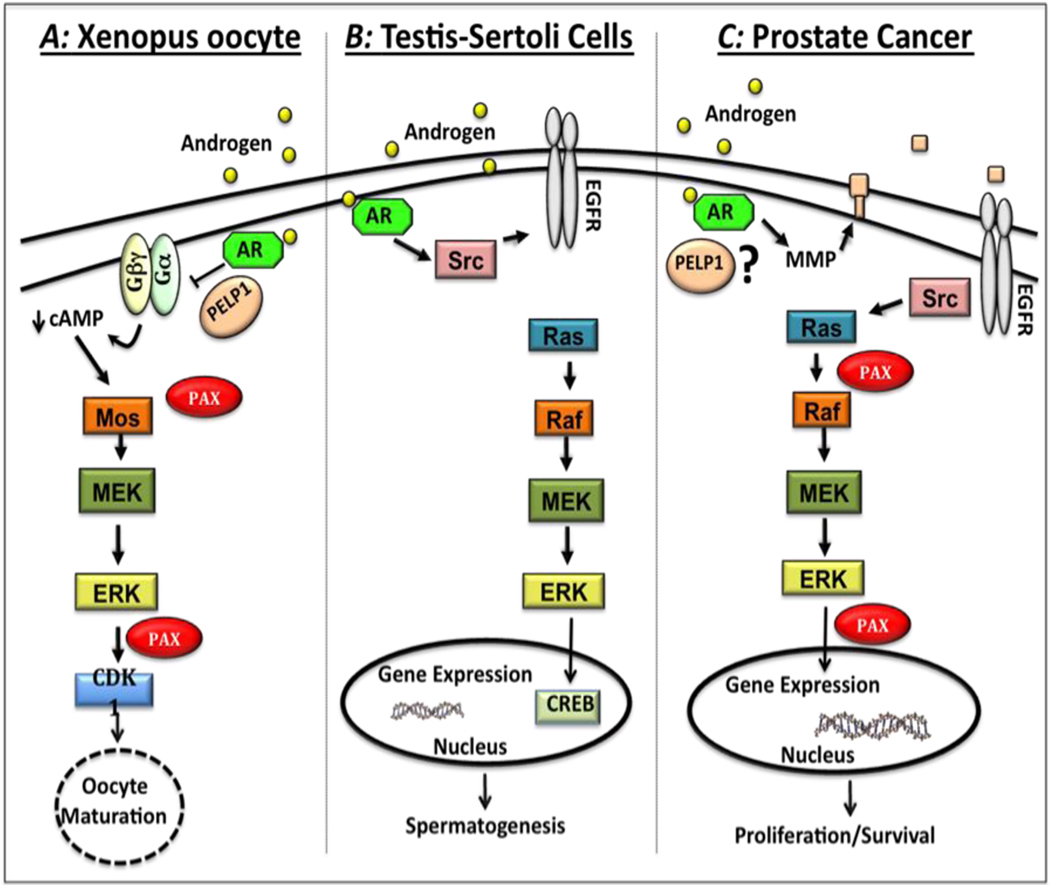
Figure 1. Comparison of androgen-induced signaling pathways in Xenopus oocytes, Sertoli cells, and prostate cancer cells.
A) In Xenopus oocytes, testosterone binding to classical androgen receptors (ARs) cause a conformational change in the AR-PELP1-Gβγ complex that suppresses G-protein mediated constitutive signaling, leading to decreased intracellular cAMP levels. Once cAMP levels drop, downstream kinases are activated, starting with a small increase in MOS protein expression. MOS in turn activates the MEK-Erk pathway. Activated Erk phosphorylates paxillin, which in turn leads to increased MOS protein expression and more Erk activation. This positive feedback loop ultimately leads to activation of cyclin dependent kinase CDK1 and subsequent oocyte maturation. B) In Sertoli cells, testosterone binds to classical ARs leading to activation of Src that promotes phosphorylation of the epidermal growth factor receptor (EGFR), resulting in activation of the MAPK pathway (Raf-MEK-Erk). Once Erk is phosphorylated, it activates the kinase p90RSK, which promotes phosphorylation of the cAMP Response Element Binding (CREB) protein leading to increased transcription. C) In prostate cancer cells, androgen induces transactivation of the EGFR via matrix metalloproteinase (MMP)-mediated release of membrane-bound EGFR ligands. EGFR activation leads to Src-mediated phosphorylation of paxillin that is essential for subsequent activation of Ras/Raf/MEK/Erk pathway. Activated Erk in turn phosphorylates paxillin that regulates Erk-mediated downstream processes such as intranuclear transcription, proliferation, cell survival and migration.