Figure 3.
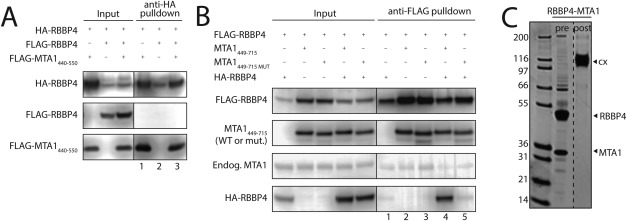
The C‐terminal half of MTA1 can bind two molecules of RBBP4. A. Immunoprecipitation analysis showing that MTA1440–550, which encompasses a single RBBP‐binding motif (RBM) at positions 493–498 (493WHAARH), is able to bind a single RBBP4. Cleared cell lysates (Input) of HEK293 cells expressing the proteins indicated on top, as well as anti‐HA immunoprecipitates (anti‐HA pulldown), were analysed by Western Blot using anti‐HA or anti‐FLAG antibodies, to detect the proteins indicated on the left. The absence of FLAG‐RBBP4 in the elution of the triple co‐transfection (lane 3) suggests that only one RBBP4 can bind to MTA1440–550. B. Immunoprecipitation analysis showing that MTA1449‐715, which contains the two RBMs at positions 493–498 and 678–683, is able to bind two molecules of RBBP4. Cleared cell lysates (Input) of HEK293 cells expressing the proteins indicated on top, as well as anti‐FLAG immunoprecipitates (anti‐FLAG pulldown), were analysed by Western Blot using anti‐FLAG, anti‐HA or anti‐MTA1 antibodies, to detect the proteins indicated on the left. The results show that FLAG‐RBBP4 can efficiently pull down HA‐RBBP4 in the presence of MTA1449–715, suggesting that this fragment can bind two molecules of RBBP4. However, this effect is not observed when using an MTA1449–715 version mutated at one of the RBMs (678KRAARR to AAAAAA). C. Sypro‐stained SDS‐PAGE showing purified RBBP4‐MTA1449–715 before and after treatment with glutaraldehyde. The crosslinked complex runs at a molecular weight consistent with the formation of a 2:1 (RBBP4:MTA1449–715) complex.
