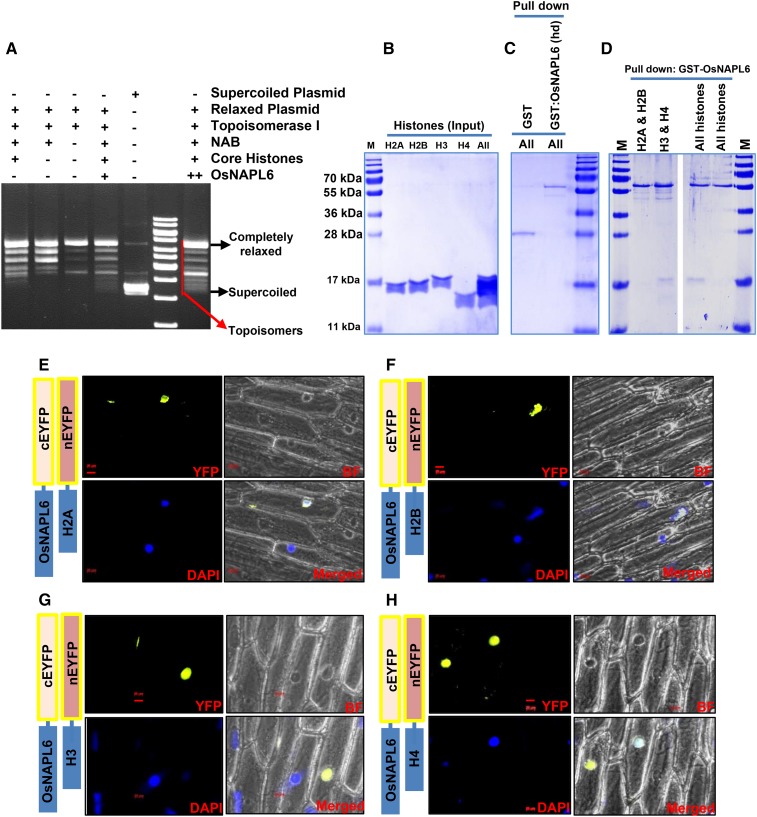
Figure 2.
OsNAPL6 is a functional H3/H4 histone chaperone capable of assembling nucleosomes in vitro. A, A plasmid supercoiling assay was utilized for analyzing the nucleosome assembly activity of OsNAPL6 where supercoiled plasmid DNA was first relaxed with Topoisomerase I and the preincubated histone octamer-OsNAPL6 complex was added to the prerelaxed plasmid. Nucleosome-like structures were allowed to form on the relaxed plasmid template in the presence of Topoisomerase I. The proteins were digested and the DNA extracted. Supercoiling of the prerelaxed plasmid DNA resulted in topoisomers with different linking numbers. These topoisomers were separated on a 1.2% agarose gel and the gel was stained with ethidium bromide, postrunning. The positions of completely supercoiled and relaxed forms are indicated. NAB, Nucleosome assembly buffer. B to D, GST pull-down assay for analyzing the histone binding specificity of OsNAPL6. GST-OsNAPL6 fusion protein was immobilized onto glutathione-sepharose beads. Four histones in different combinations (H2A/H2B-H3/H4 [All], H3/H4 [H3 & H4], and H2A/H2B [H2A & H2B]) were added to the bead bound GST-OsNAPL6. Nonspecific binding was removed by multiple washing and the specifically bound histones were eluted. B, Input of histones used for each pull-down. C and D, The pull-down fractions were analyzed on a 15% SDS-PAGE gel. As a negative control, GST was checked for its activity to pull down the histones (C, first lane). To ensure specificity, heat denatured (hd) GST-OsNAPL6 fusion protein was analyzed for its ability to pull down the histones (C, second lane). “All” indicates all four core histones. E to H, BiFC assay was carried out to check the interaction of OsNAPL6 with various histones in planta. OsNAPL6 and H2A (E), H2B (F), H3 (G), or H4 (H) cloned in complementary split-YFP vectors were cotransformed into onion peel epidermal cells using biolistic bombardment method, as indicated. After 16 h of incubation at 28°C, the peels were stained with nuclear marker 4′,6-diamino-phenylindole (DAPI) and observed under a fluorescence microscope. Bars placed at the bottom of the micrographs represents 20 μm. BF, Bright field. See also Supplemental Figure S2.