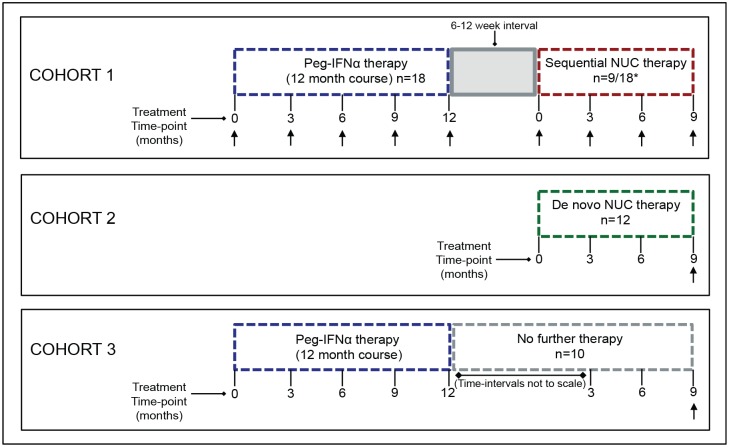
Fig 1. Schematic overview of patients studied for immune analysis.
Cohort 1 indicates sequential NUC therapy patients studied; 18 consecutive patients were analysed longitudinally during a 48 week course of PegIFNα therapy (blue dashed outline), of which 9/18 patients progressed to sequential NUC therapy, following a 6–12 week interval gap (grey shaded box), and were sampled longitudinally; *indicates 5 further patients analysed undergoing sequential NUC therapy, sampled at time-point month 0 and 9 only on sequential therapy (total sequential NUC therapy cohort; n = 14) (red dashed outline). Cohort 2; n = 12 patients analysed at a single time-point at viral suppression on de novo NUC therapy (green dashed outline). Cohort 3; n = 10 patients treated with PegIFNα therapy for 48 weeks that did not undergo any further treatment (grey dashed outline), and sampled at 9 months following cessation of PegIFNα. Arrows under time-points indicate sampling time for immune analysis in each cohort.