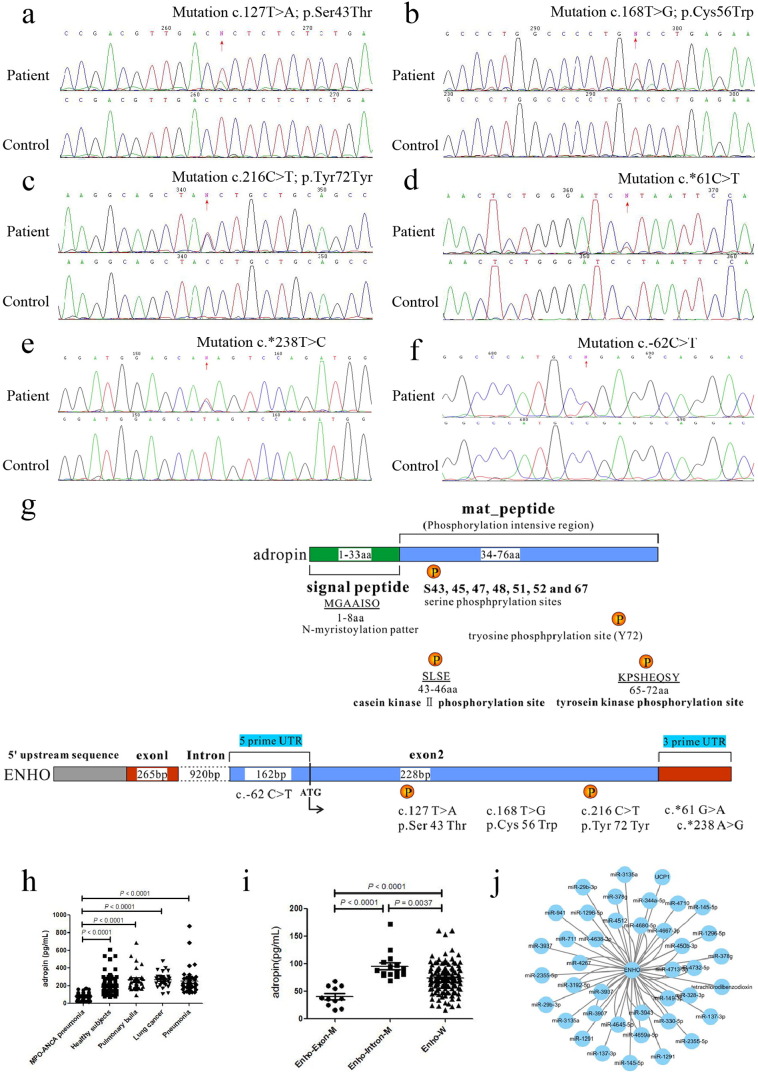
Fig. 1.
Mutations of Enho in MPO-ANCA associated lung injury.
(a) c.127T > A (p.Ser43Thr) mutation. (b) c.168T > G mutation result in p.Cys56Trp amino acid substitution. (c) c.216C > T (p.Tyr72Tyr) mutation. (d) c.*61T > C mutation. (e) c.*238T > C mutation. (f) c.-62C > T mutation. (g) Top panel shows scheme of the adropin domain structure. Adropin contains a signal peptide domain (1-33aa, green), and a mat_peptide domain (34-76aa, light blue) which containing predicted serine phosphorylation sites (S43, 45, 47, 48, 51, 52 and 67) and tyrosine phosphorylation site (Y72). Using the Motif Scan program, there are an N-myristoylation patter (MGAAISQ), a casein kinase II phosphorylation site (positions 43–46, SLSE), and a tyrosine kinase phosphorylation site (positions 65–72, KPSHEQSY). Bottom panel shows scheme of the location of Enho mutations causing MPO-ANCA-related pneumonia. (h) The serum concentration of adropin in patients with MPO-ANCA-related pneumonia (MPO-ANCA associated lung injury), healthy subjects, pulmonary bulla, lung cancer, and pneumonia. (i) Serum concentration of adropin in MPO-ANCA associated lung injury patients with different Enho genotypes. (j) MicroRNA-mRNA interaction network of Enho.