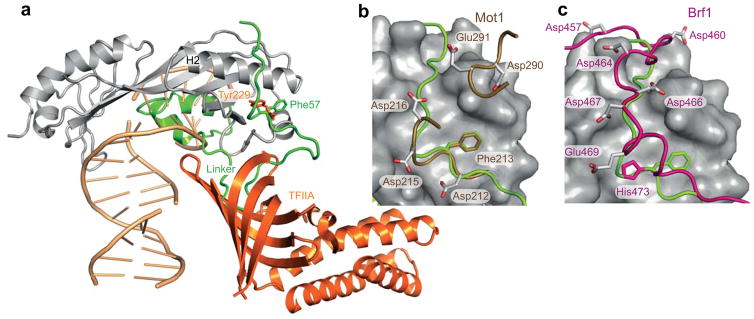
Figure 6. Conserved surface groove and anchoring residues in competitive TBP binding.
(a) Superposition of TBP-TAF1 on to the TBP-TFIIA (orange) –DNA (wheat) ternary complex (from PDB 1NH2). Aromatic TBP-anchoring residues of TAF1 and TFIIA on the convex TBP surface are shown as sticks and labeled. The linker region between TAND1 and TAND2 of TAF1 protruding into the space occupied by the β-barrel TFIIA structure is highlighted. (b) Mot1 (sand, PDB 3OC3) and (c) Brf1 (magenta, PDB 1NGM) complexes with TBP superimposed onto yTAF1 (green) – yTBP (surface), highlighting in stick representation the common anchoring aromatic residue in these transcription activators and repressors as well as hydrogen binding sidechains connecting to the basic region of TBP. All superpositions were made by structural alignment of the TBP backbone in the respective complexes onto yTBP in the current structure. Only yTBP from the current structure is shown in a–c.