Figure 1. Variations on a theme: the mitochondrial pathway of apoptosis.
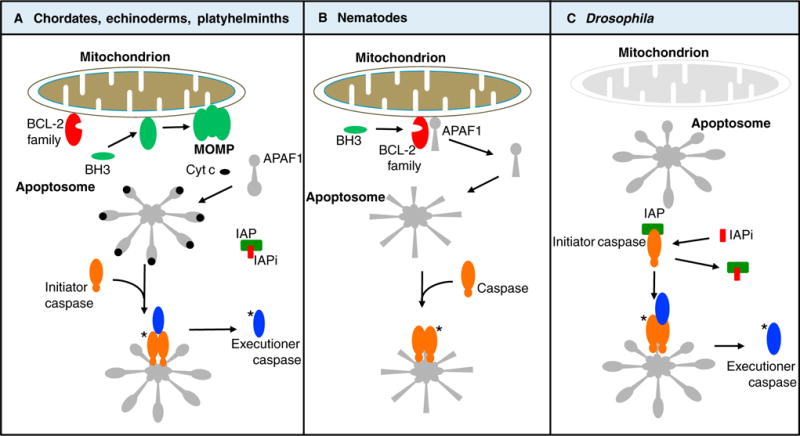
(A) In chordates, echinoderms, platyhelminths, and possibly other phyla, apoptotic conditions engage the members of the Bcl-2 family, which either promote (green) or inhibit (red) MOMP in response to activation of BH3-only proteins. MOMP results in the release of cytochrome c (cyt c) and other proteins of the intermembrane space. Cytochrome c binds to APAF1, which oligomerizes to create the apoptosome that in turn binds to and activates an initiator caspase. The initiator caspase cleaves and thereby activates executioner caspases. Both the initiator and executioner caspases are inhibited by an inhibitor of apoptosis protein (IAP). MOMP releases antagonists of the IAP (IAPi), permitting the executioner caspases to orchestrate apoptosis. Asterisks (*) refer to active caspases. (B) In nematodes, the anti-apoptotic Bcl-2 protein sequesters the APAF1 homolog. Apoptotic conditions induce expression of BH3-only proteins, which bind to the Bcl-2 protein, releasing the APAF1 homolog to form an apoptosome. This binds and thereby activates the caspase to promote apoptosis. (C) In Drosophila, the APAF1 homolog appears to spontaneously form an apoptosome, but the initiator caspase is inhibited by an IAP. Apoptotic conditions promote expression of IAPi, permitting the apoptosome to now activate the initiator caspase, which in turn cleaves and thereby activates executioner caspases, promoting apoptosis. Note that MOMP, upstream of caspase activation, occurs only in the pathway shown in (A).
