Abstract
Cholesterol modulates the bilayer structure of biological membranes in multiple ways. It changes the fluidity, thickness, compressibility, water penetration and intrinsic curvature of lipid bilayers. In multi-component lipid mixtures, cholesterol induces phase separations, partitions selectively between different coexisting lipid phases, and causes integral membrane proteins to respond by changing conformation or redistribution in the membrane. But, which of these often overlapping properties are important for membrane fusion? – Here we review a range of recent experiments that elucidate the multiple roles that cholesterol plays in SNARE-mediated and viral envelope glycoprotein-mediated membrane fusion.
Keywords: cholesterol, membrane fusion, virus entry, exocytosis, fusion protein, fusion peptide, SNARE, viral envelope protein
Graphical abstract
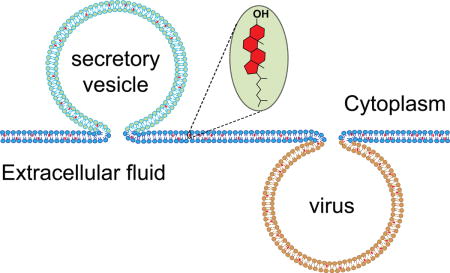
Introduction
Cholesterol is an essential component of mammalian cells. It is synthesized in a complex series of enzymatic steps in the endoplasmic reticulum and is eventually transported through the Golgi to the plasma membrane where its concentration is much higher than in other cellular compartments. Large reservoirs of cholesterol also reside in blood serum in the form of lipoproteins, which are taken up by cells through endocytosis and recycled into the intracellular pool of cholesterol. Thus cholesterol cycles within cells and in and out of cells with many of these transport functions involving fission and fusion between different membranes. Because cholesterol has profound physical effects on the membranes in which it resides, it is not surprising that membrane cholesterol also dramatically affects membrane fusion and membrane fission. In this review, we first recapitulate briefly some of the unique effects that cholesterol imparts on the host lipid bilayer and some common modes of how cholesterol interacts with integral membrane proteins. This sets the stage to discuss a host of relatively recent discoveries on how cholesterol influences membrane fusion in intracellular membrane traffic, particularly in exocytosis of secretory vesicles, and in cell entry of enveloped viruses whose membranes typically are also highly enriched in cholesterol.
Cholesterol orders lipids and induces phase separation and curvature changes in fluid lipid bilayers
Cholesterol has a unique structure of four fused hydrocarbon rings with a polar hydroxyl group at one end and an eight-carbon branched aliphatic tail at the other end. The ring structure is rigid with an almost flat front face and a more corrugated back face, whereas the tail is flexible and able to undergo trans-gauche isomerizations like the hydrophobic tails of the phospholipids of the bilayer in which cholesterol resides. The small hydroxyl group is the only polar group in the molecule; the remainder is highly apolar and therefore deeply immersed in the host lipid bilayer.
The polar hydroxyl group aligns approximately with the ester carbonyl groups of the phospholipids in which it is perpendicularly embedded (Smondyrev and Berkowitz, 1999; Heftberger et al., 2015). The rigid ring structure considerably reduces the transgauche isomerizations of the neighboring lipid acyl chains and therefore orders them and reduces their dynamics and fluidity. Hence, this mixed phospholipid/cholesterol phase has been termed a liquid-ordered (Lo) phase (Ipsen et al., 1989; Sankaram and Thompson, 1990), in contrast to the liquid-disordered (Ld) phase of phospholipid bilayers in the absence of cholesterol above their chain melting phase transition. The ordering effect of cholesterol on fluid lipid bilayers does not only reduce the dynamics of the individual lipids, it also alters the continuum properties of the lipid bilayer like bending and compressibility moduli (Chen and Rand, 1997). The increased viscosity of cholesterol-containing membranes slows down the lateral and rotational diffusion of lipids and embedded membrane proteins (Rubenstein et al., 1979; Kahya et al., 2003; Crane and Tamm, 2004). A summary of mutlple effects that cholesterol can exert on membranes and membrane proteins is shown in Figure 1.
Figure 1.
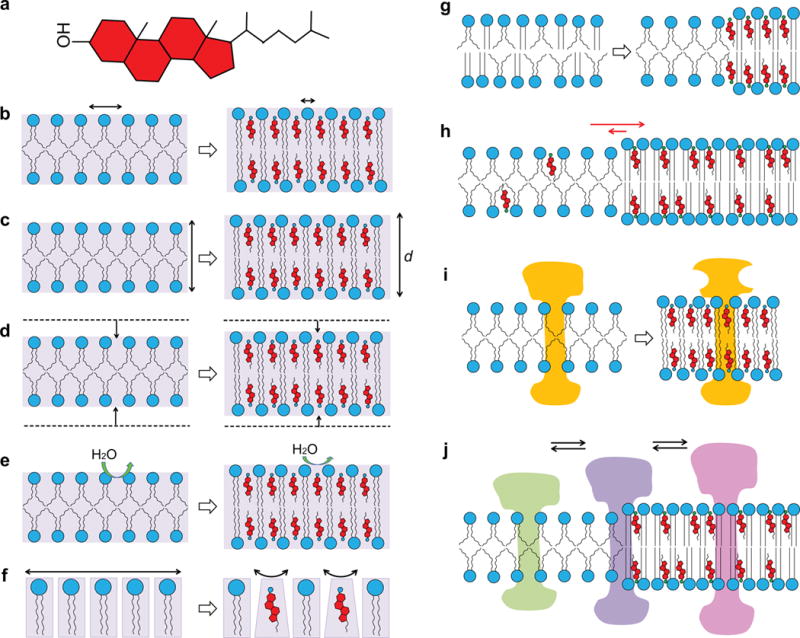
Cholesterol has multiple effects on lipid bilayers. Cholesterol (a) changes the fluidity (b), thickness (c), compressibility (d), water penetration (e), and intrinsic curvature (f) of lipid bilayers. Cholesterol also induces phase separations in multicomponent lipid mixtures (g), partitions selectively between different coexisting lipid phases (h), and causes integral membrane proteins to respond by changing conformation (i) or redistribution (j) in the membrane.
In multi-component lipid mixtures, cholesterol interacts preferentially with long, saturated phospho- and sphingo-lipids, which on their own exhibit high gel-to-liquid-crystalline phase transition temperatures. Therefore, lipid bilayers composed of a low- and a high-melting lipid and cholesterol laterally segregate over a wide range of proportions into cholesterol-rich Lo and cholesterol-poor Ld domains (Brown and London, 1998; Feigenson, 2006). Such domains, termed lipid rafts and enriched in cholesterol and sphingolipids, are hypothesized to also exist in the membranes of living cells (Simons and Ikonen, 1997; Lingwood and Simons, 2010) where they seem to be small, dynamic and transient, which makes their study difficult and controversial (Shaikh and Edidin, 2006). In model membrane systems, some of the factors that lead to submicroscopic, dynamic domains in cells can be eliminated so that phase separation occurs on a larger scale, exhibiting micrometer-size domains that can be easily observed by fluorescence microscopy. Lo phase domains surrounded by continuous Ld phase lipid bilayers can be used as simple models for lipid rafts, which permit studies of the relevant physical interactions under controlled conditions (Veatch and Keller, 2005; Feigenson, 2006; Jacobson et al., 2007; Crane and Tamm, 2007; Sezgin et al., 2012; Kiessling et al., 2015).
Cell membranes are highly asymmetric in terms of lipid composition. Phospho- and sphingo-lipid, but not cholesterol flip-flop across the membrane is slow in the absence of “flippases” in healthy, non-apoptotic cells. Such lipid asymmetry can also be generated and maintained in some model systems with and without lipid rafts (Crane et al., 2005; Garg et al., 2007; Lin and London, 2014). It has been shown that sphingomyelin-rich domains in the outer leaflet of the plasma membrane can couple and induce domains in the inner leaflet of the membrane, which otherwise might not be present (Kiessling et al., 2006; Collins and Keller, 2008; Wan et al., 2008; Kiessling et al., 2009; Chiantia et al., 2011; Wan et al., 2011). All intracellular membrane fusion processes take place by initial contact of inner leaflet lipids and thus induced phase heterogeneity in these leaflets of the membrane may be mechanistically important.
Since cholesterol straightens out the lipid tails in cholesterol-rich Lo phase lipid bilayers, such bilayers are usually thicker than cholesterol-poor Ld phase bilayers (Pan et al., 2008). When both phases coexist in membranes, a discontinuity of the bilayer width arises at the phase boundary, which results in a line-tension that has direct implications on membrane curvature at the interface between the Lo and Ld phases (Baumgart et al., 2003). Line-tension at Lo-Ld interfaces has been recognized as an essential parameter that controls the kinetics of phase separation and the sizes of lipid domains (Garcia-Saez et al., 2007). Since line-tension also induces membrane bending it can drive the budding and formation of new vesicles at domain boundaries (Julicher and Lipowsky, 1993; Garcia-Saez et al., 2007).
Due to the very small size of the polar headgroup compared to the cross-sectional area of the apolar portion, cholesterol generates intrinsic negative curvature in lipid bilayers. Cholesterol thereby has the potential of promoting highly curved membrane structures such as lipid stalks that have been proposed as lipid intermediates in membrane fusion (Yang and Huang, 2002; Chernomordik and Kozlov, 2008; Aeffner et al., 2012). The resistance that lipid bilayers exhibit towards bending into curved structures that are different from their equilibrium structure is expressed in the curvature elasticity and is dependent on the lipid composition. Cholesterol increases the bending modulus and therefore the stiffness of fluid membranes, especially when they consist of saturated lipids and are in a Lo phase state (Evans and Rawicz, 1990; Pan et al., 2009).
Cholesterol modulates the structure and activity of integral membrane proteins by different mechanisms
Cholesterol influences the behavior of membrane proteins in lipid bilayers in multiple ways (Epand, 2008). Generally, we can distinguish between (i) global effects of the perturbed lipid bilayer, discussed in the previous section, on membrane protein behavior and (ii) specific effects of cholesterol binding to defined binding motifs on membrane proteins. The increased order of the lipid acyl chains results in a reduction of free volume in bilayers when cholesterol is introduced (Falck et al., 2004). This increased free volume changes the conformational behavior and shifts conformational equilibria of membrane proteins in the presence of cholesterol. These effects have been extensively studied with G-protein-coupled receptors (GPCRs), most notably how cholesterol affects the Meta I – Meta II equilibrium in the photocycle of rhodopsin (Niu et al., 2002; Bennett and Mitchell, 2008; Paila and Chattopadhyay, 2010; Soubias and Gawrisch, 2012). In other GPCRs, e.g. the oxytocin, cholecystokinin, β2-adrenergic, and serotonin 1A receptors, cholesterol enhances ligand binding and downstream signaling (Gimpl et al., 1997; Gimpl and Fahrenholz, 2002; Hanson et al., 2008; Saxena and Chattopadhyay, 2012).
Cholesterol stabilizes the structure of the M2 proton channel in the influenza envelope membrane. The length of the transmembrane (TM) domain of the M2 protein is relatively short preferring relatively thinner (Ld) regions of the membrane, but the amphipathic helix of M2 is stabilized by higher concentrations of cholesterol present in thicker (Lo) regions of the membrane (Ekanayake et al., 2016). To satisfy both requirements M2 prefers Lo domains, but may partition into domain boundary regions where it could help viral budding from the cell membrane envelope. Indeed, both M2 and cholesterol are needed for efficient budding of influenza particles from virus producing cells (Rossman et al., 2010). Binding of the anti-influenza drug amantadine in the pore of the tetrameric M2 channel is independent of cholesterol (Ekanayake et al., 2016).
Several binding motifs have been identified for specific binding of cholesterol to membrane proteins (Fantini and Barrantes, 2013). These motifs share three main properties. First, they contain a basic residue (K or R) proposed to bind the hydroxyl group of cholesterol; second, they share an aromatic residue (Y, F, or W) proposed to interact with the rings of cholesterol; and third, they contain one or more aliphatic residue (I, L, or V) proposed to interact with the aliphatic tail of cholesterol by van der Waals forces. The best documented cholesterol-binding motif is the cholesterol recognition amino acid consensus (CRAC) motif. The CRAC motif is characterized by the linear sequence (L/V)-X1–5-(Y)-X1–5-(K/R) in the single letter amino acid code. The direction of this motif is critical and starts from N-terminus to C-terminus. For example, a CRAC motif in the peripheral-type benzodiazepine receptor binds and thereby facilitates the uptake of cholesterol (Li et al., 2001; Jamin et al., 2005). CRAC moifs have been reported to occur in multiple GPCRs (Jafurulla et al., 2011; Oddi et al., 2011). A CRAC motif is also present and functionally important just before the TM domain of the surface glycoprotein gp41 of HIV (Vishwanathan et al., 2008). The substitution and deletion mutagenesis of this CARC motif (LWYIK) reduced the fusion efficiency and the first cycle of viral replication (Chen et al., 2009).
The reverse directional cholesterol-binding motif is called CARC motif defined as (K/R)-X1–5-(Y/F)-X1–5-(L/V) (Baier et al., 2011). In addition to the different polarity, the CARC motif can have a central Y or F, whereas it is a universal Y in the CRAC motif. However, in each case variable numbers of variable amino acids (X) can be placed between the central and flanking key residues.
The cholesterol consensus motif (CCM) is found in TM domains of polytopic membrane proteins and depends on the spatial arrangement rather than the linear sequence of the participating residues, which are distributed between two TM domains. The first TM domain contains typical cholesterol-binding residues (K/R)(W/Y)(I/V/L) facing the same side of the TM helix. Cholesterol binding is then stabilized by an aromatic residue (Y or F) on an apposed face of the second TM helix, which interacts with the other side of cholesterol. This motif occurs in the β2-adrenergic receptor (Hanson et al., 2008) and has also been found in influenza hemagglutinin (de Vries et al., 2015). Remarkably, the cholesterol-binding site in the adrenergic receptor is adjacent to a therapeutic drug targeting site.
Additional aspects of membrane proteins may also introduce cholesterol-binding sites such as a tilted helix implicated in α-synuclein (Fantini and Yahi, 2011), the HIV fusion peptide (Fantini and Barrantes, 2013), and the GXXXG motif found in the amyloid precursor protein (Barrett et al., 2012), but these cases are less well documented.
Effect of cholesterol on SNARE-mediated intracellular membrane fusion
Regulated exocytosis is a fundamental biological process where secretory vesicles release cargo products (neurotransmitters, peptides, hormones etc.) into the extracellular space by a process during which the vesicle membrane fuses with the plasma membrane (Rothman, 2014) (Figure 1). SNARE (Soluble NSF Attachment Protein Receptor) proteins are at the core of a molecular machinery that leads to pore opening and secretory content release (Tamm et al., 2003; Rothman, 2014). The membrane composition of secretory cells appears to be highly tuned to respond rapidly to stimuli that catalyze the membrane fusion reaction. The plasma membrane and secretory vesicles contain ~30–40% cholesterol. Cholesterol has been shown to be necessary for exocytosis in neuronal (Wasser et al., 2007; Linetti et al., 2010), endocrine (Hao and Bogan, 2009), and neuroendocrine cells (Koseoglu et al., 2011; Zhang et al., 2009), as well as in cortical vesicles from sea urchins (Churchward et al., 2005; Churchward et al., 2008). Manipulating the cholesterol content by removal with methyl-β-cyclodextrin (Churchward et al., 2005; Koseoglu et al., 2011) or by deletions of proteins involved in cholesterol homeostasis (Sturek et al., 2010; Kruit et al., 2011) greatly impairs secretory content release. Many of the above-discussed general effects that cholesterol imparts on membrane structure and dynamics have the potential to affect regulated exocytosis. For example, it is known that cholesterol clusters SNAREs in the plasma membrane of neuroendocrine cells (Lang et al., 2001) and it has been postulated that synaptic vesicle SNAREs may reside in raft-like regions of the vesicle membrane (Chamberlain et al., 2001; Gil et al., 2005). However, at least for the target membrane SNAREs synatxin-1a and SNAP-25, it has been shown that the clusters, although cholesterol-dependent, are not lipid rafts (Lang, 2007). As discussed by Destainville et al. (Destainville et al., 2016) recent advances in super resolution microscopy have shown that syntaxin-1a exists in 50–60 nm sized clusters containing ~75 syntaxin-1a molecules (Sieber et al., 2007; Knowles et al., 2010) and that SNAP-25 exists in similar sized clusters of about ~70 molecules (Knowles et al., 2010).
Results from reconstitution of SNAREs into model membranes have shown several ways by which cholesterol may promote the clustering of SNAREs in target membranes (Figure 2). The neuronal plasma membrane SNARE syntaxin-1a may be more soluble in Ld than in Lo phase membranes (Bacia et al., 2004) because of different lipid ordering (Murray and Tamm, 2009) or because of hydrophobic mismatch (Milovanovic et al., 2015). Interestingly, the cholesterol-dependent clustering of syntaxin-1a is further modulated by electrostatic interactions with negatively charged lipids including phosphatidylserine (PS) and phosphatidylinositol-(4,5)-bisphosphate (PIP2) (Murray and Tamm, 2009, 2011; van den Bogaart and Jahn, 2011). The polybasic juxta-membrane domain of syntaxin-1a is responsible for interactions with acidic lipids (Murray and Tamm, 2011). Whether PIP2 breaks up clusters of syntaxin (Murray and Tamm, 2009, 2011) or forms them (van den Bogaart and Jahn, 2011) is still debated.
Figure 2.
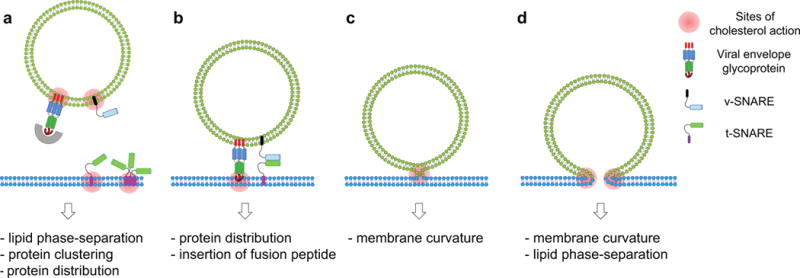
Possible contributions of cholesterol to the regulation of membrane fusion proteins and fusion intermediates and to overcoming multiple energetic barriers of membrane fusion. The schematic diagrams illustrate a protein-assisted stalk-pore model of membrane fusion mediated by SNARE or viral envelope glycoproteins. (a) Secretory vesicles or enveloped viruses are targeted to docking and fusion sites. Cholesterol may influence lipid phase-separation, protein distribution and protein clustering, thereby affecting the docking step. (b) Interaction of at least some viral fusion peptides with the target membrane appears to be dependent on cholesterol. For example, cholesterol-mediated microdomains or clusters may facilitate the insertion of fusion peptides at domain boundaries. (c) Cholesterol can lower the energy for lipid stalk formation by providing intrinsic negative curvature. (d) The ordered structure and negative intrinsic membrane curvature of cholesterol-rich regions opposes fusion pore formation (unless cholesterol is asymmetrically distributed to the distal leaflets of the fusing membranes), but boundaries of lipid rafts and protein clusters induced by cholesterol may promote fusion pore formation in some cases. The multiple roles of cholesterol in the individual fusion steps may be oversimplified in these cartoons, but the main point is that cholesterol affects membrane fusion in multiple complex ways.
In addition to the raft-independent cholesterol-mediated clustering of SNAREs, cholesterol-rich nanoscopic lipid rafts may have other roles in secretory vesicle fusion. For instance, using asymmetric supported membranes, Wan et al (Wan et al., 2011) showed that the anionic lipids PS and PIP2 of the inner plasma membrane leaflet, which are essential for calcium-triggered membrane fusion, selectively partition between Lo and Ld membrane domains in phase-separated membranes. The C2 domains of the calcium sensor synaptotagmin 1 are thereby directed to bind in a calcium-dependent fashion to the less ordered Ld regions of the membrane, that accumulate more PIP2 than the Lo regions.
Owing to its intrinsic negative curvature, cholesterol has been proposed to lower the energy for forming lipid stalks that are believed to be intermediates in membrane fusion and to also stabilize fusion pores (Churchward et al., 2005; Churchward et al., 2008). Cholesterol’s direct effects on the dynamics of forming fusion pores has been measured in cells using amperometery (Ge et al., 2010; Wang et al., 2010; Koseoglu et al., 2011; Gruba et al., 2015; Finkenstaedt-Quinn et al., 2016). Recapituating these effects observed in cells, reconstitution of SNARE-mediated fusion in different biochemical settings also shows more efficient fusion when the cholesterol content is increased in the membrane (Tong et al., 2009; Kreutzberger et al., 2015; Stratton et al., 2016). Even in protein-free fusion, increasing cholesterol increases membrane fusion (Lee et al., 2013).
Recent studies measuring fusion at the single vesicle level showed that cholesterol might directly modulate the formation and stability of fusion pores (Kreutzberger et al., 2015; Stratton et al., 2016). Using planar supported bilayers with SNAREs reconstituted in an oriented and laterally mobile fashion Kreutzberger et al. (Kreutzberger et al., 2015) found that a majority of vesicles undergo hemi-fusion in the absence of cholesterol, but when cholesterol was increased to physiological levels of 30–40 mol% in the target or vesicle membrane most vesicles underwent direct full fusion bypassing a long-lived (>2–4 milliseconds) hemifusion intermediate. Replacing cholesterol with α-tocopherol, a molecule with similar intrinsic curvature as cholesterol, showed a very similar behavior of fusion efficiency as a function of concentration, providing strong evidence that the property of cholesterol to change membrane curvature is likely the main mechanism responsible for the observed behavior (Kreutzberger et al., 2015). Using a similar assay, Stratton et al. (Stratton et al., 2016) confirmed that cholesterol decreases the energy barrier for fusion pore formation.
CARC and CRAC motifs have been identified in several, but by no means all (actually in 11 of 38 human) SNARE proteins (Enrich et al., 2015). Their presence or absence does not seem to correlate with the cholesterol content of the membrane in which these SNAREs reside, raising questions on their functional relevance. For example, the plasma membrane SNAREs syntaxin 1, 2, 3 and 4 do not have distinctive cholesterol binding motifs although that membrane contains larger concentrations of cholesterol than intracellular membranes. Tong et al. (Tong et al., 2009) showed that increases of cholesterol in reconstituted vesicle SNARE synaptobrevin membranes stimulated fusion measured by lipid mixing more than an increase of cholesterol in the target SNARE membranes. These authors also reported that the synaptobrevin TM domain was tilted in the absence of cholesterol, but became more perpendicular to the membrane plane in the presence of cholesterol (Tong et al., 2009). The partitioning of the SNARE motif of synaptobrevin between the membrane surface and solution (Liang et al., 2014) is also likely increased towards the solution conformation at higher cholesterol, which would also explain the enhanced fusion that Tong et al. observed in the presence of cholesterol.
Effect of cholesterol on membrane fusion in enveloped virus entry
Membrane fusion is a key step of enveloped virus entry into host cells (Zimmerberg et al., 1993; Blumenthal et al., 2003; Harrison, 2008) (Figure 1). While viral surface glycoproteins drive membrane fusion, lipids including cholesterol play critical roles in the fusion process (Chernomordik and Kozlov, 2003; Tamm et al., 2003; Lai et al., 2005) (Figure 2). A growing body of evidence supports the idea that cholesterol-rich regions serve as platforms for the entry of many enveloped viruses (Manes et al., 2003). The cholesterol requirement in virus entry has been evaluated by the inhibition of infection after cholesterol depletion from virus and/or host membranes by methyl-β-cyclodextrin (MβCD). Some viruses like the human immunodeficiency virus (HIV) require cholesterol on both viral and target membranes for infection (Liao et al., 2001; Graham et al., 2003) whereas others including the influenza virus require cholesterol only in the viral membrane (Sun and Whittaker, 2003). In either case, cholesterol depletion significantly impairs viral entry, but has little effect on viral binding to host cells, indicating that cholesterol is crucial for membrane fusion.
The non-random distribution of proteins and lipids in cell membranes extends to viral membranes (Simons and Ikonen, 1997; van Meer et al., 2008). It is believed that many viruses including HIV and influenza virus exploit lipid rafts for assembly and budding (Scheiffele et al., 1999; Freed, 2015). In fact, many viral membrane envelopes contain more cholesterol than typical mammalian plasma membranes from which they are derived. Consequently, lipid mixtures mimicking the HIV envelope exhibit a large fraction of raft-like Lo domains in model membranes and show a distinct lipid phase behaviour that depends on the concentration of cholesterol (Yang et al., 2015). The area fraction of Lo domains increases linearly with the cholesterol concentration. Historically, proteins suspected to be associated with lipid rafts have been identified often by their localization in detergent-resistant membrane (DRM) fractions, i.e., membrane fractions that are enriched in sphingolipids and cholesterol and that are isolated at low temperature after detergent extraction from cells. Using these criteria, the envelope glycoproteins of HIV, gp120/gp41 and of influenza virus, hemagglutinin (HA) were found to be associated with lipid rafts (Scheiffele et al., 1997; Waheed and Freed, 2009). Their lateral distribution in the viral envelope is thought to be influenced by the local cholesterol concentration (Sun and Whittaker, 2003). However, details about the spatial organization of envelope proteins on the virion surfaces and their relation to lipid composition, except for rare cases (Domanska et al., 2015), has not been examined in great detail.
A direct influence of cholesterol on the fusion efficiency in HA-mediated fusion has been observed in vitro (Biswas et al., 2008; Domanska et al., 2013). While an observed decrease of the fusion rate upon cholesterol depletion may likely be the result of a lesser stabilization of a lipidic fusion intermediate in the absence of negative curvature-promoting cholesterol (Domanska et al., 2013), an increase of fusion rates that was observed at moderate cholesterol depletion of viral particles was explained by the closer proximity and thus enhanced cooperativity of multiple HAs contributing to each fusion event (Domanska et al., 2015). Severe cholesterol depletion likely also increases the fraction of gel phase of the remaining lipids, which likely hinders fusion by the reduced lateral mobility of the HAs and by the mechanical properties of such bilayers that are likely unfavorable for fusion.
It is well established that fusion peptides of viral fusion proteins play a critical role in membrane fusion via their direct insertion into the host membranes (Tamm et al., 2002; Epand, 2003; Tamm et al., 2014). The fusion peptide of HIV (HIV-FP) has been shown to change secondary structure depending on the cholesterol content in the membrane (Lai et al., 2012). In the absence of cholesterol the HIV-FP adopts a mostly α-helical conformation in lipid bilayers, but it increasingly shifts to a β-structure conformation as the membrane cholesterol is increased. Both the fully α-helical and the fully β-structured FPs have the ability to insert relatively deeply into the lipid bilayer, whereas the mixed secondary structures that were observed at intermediate cholesterol concentration were more shallowly inserted into lipid bilayers. Interestingly, both deeply inserted structures promoted rapid and efficient lipid mixing of liposomes, but the more shallowly inserted mixed structures were less effective at inducing liposomal membrane fusion (Lai et al., 2012).
The above described results raise the interesting possibility that different secondary structures might be important at different stages in fusion and that cholesterol-rich and cholesterol-poor membrane regions may be sequentially involved in fusion. Indeed, Yang et al. (Yang et al., 2015) found that fusion of model membranes with coexisting Lo and Ld phases is even more efficient than fusion of uniform Lo or Ld phase membranes. When HIV-FP-mediated fusion of coexisting Lo/Ld phases was analyzed at the single liposome level (or with single pseudovirions with complete HIV envelope proteins), it was found that most binding and fusion events occur at the boundaries between the raft-like Lo and the more fluid Ld domains. When multiple physical properties were examined that could all potentially contribute to these remarkable observations, it was found that line tension at the phase boundaries was the unifying principle that could explain the enhanced fusion of virion or virosomes at lipid phase boundaries (Yang et al., 2016). The line tension is reduced when, upon membrane fusion, multiple Lo domains merge into a larger domain. This reduction in line tension can provide a substantial fraction of the energy that drives fusion in this system. In addition, lipid phase discontinuities introduce membrane defects with exposed hydrophobic surfaces that are favorable for a deeper insertion of the fusion peptides and thus enhance their ability to promote membrane fusion. It would be interesting to know if the HIV-FP first assembles on cholesterol-rich membrane regions in its β-structured conformation and then migrates to the boundary where adjacent more fluid Ld phase membrane regions may convert it into its α-helical form. This conversion would allow the fusion peptide to penetrate more deeply into the lipid bilayer and thereby elicit fusion with the host membrane that may also benefit from such phase discontinuities for facilitation of fusion.
Conclusions and Future Perspectives
Despite extensive research on membrane fusion and an exhaustive literature on the effect of cholesterol on membrane structure and dynamics and on the response of numerous membrane proteins to membrane cholesterol, the intersection of fusion and cholesterol research is surprisingly small. The reasons for this are most likely (i) that many laboratories that study membrane fusion, in the SNARE and viral fusion field, focus on what the respective fusion proteins do, how they interact, and how they are regulated, while paying relatively little attention to the lipid environment in which they work and (ii) that laboratories that are focused on cholesterol often either use inadequate simple cell biological techniques like cold detergent extraction or take completely lipid-centric biophysical approaches to determine lipid phase behavior and mechanical properties of membranes with relatively little attention to complex protein machines that are embedded and work on these membranes. We believe that all these approaches by themselves are certainly very valuable and set up the correct framework to think about these problems, but that they are also incomplete when it comes to trying to understand the complex molecular mechanisms that give rise to protein-regulated membrane fusion.
However, the scenario is beginning to change. As summarized in this review, there are quite a few laboratories that are beginning to combine protein- and lipid-based approaches to understand viral and intracellular membrane fusion and many of them have turned their attention to the all important and ubiquitous cholesterol. Pictures begin to emerge that indicate that cholesterol has the ability to organize membrane fusion proteins in spatial arrangements that support the ensuing fusion process. Cholesterol has also been demonstrated to facilitate fusion by virtue of its intrinsic negative curvature-promoting ability to stabilize important curved fusion intermediates in several fusion systems and to alter secondary structure and membrane penetration depths of inserted fusogenic protein sequences. And finally, a new role for cholesterol begins to emerge, in which lateral changes in the heterogeneous fabric of membranes, such as those visible in amplified form at Lo-Ld phase boundaries, provide hot spots for membrane fusion.
Despite these significant recent advances, much remains to be done. How is cholesterol actually organized in early lipid stalk and fusion pore intermediates? How does cholesterol orchestrate the relevant proteins at the site of fusion? Or, asking the same question the other way around: how do fusion proteins attract cholesterol to the fusion zone? What other lipids and accessory proteins are also brought into this site? Is it ultimately the central role of cholesterol to provide some sort of glue and bring all the relevant players, i.e. the required lipids and proteins, into focus at the site of fusion and at the same time make the membrane complient to fusion at this focus? – Times are exciting for combining cholesterol and fusion research. Better protein structures, especially those of partially folded intermediate structures, astoundingly increased resolutions of light and electron optical approaches, and ever increasing time scales and complexities of computational approaches are on the horizon to solve these important questions. There is no shortage of opportunities to be seized to unravel the many interleaved facets of how cholesterol promotes fusion in entry of enveloped viruses and exocytosis of secretory vesicles!
Highlights.
Cholesterol is essential in fusion of secretory vesicles with plasma membranes
Cholesterol is essential in fusion in cell entry of some enveloped viruses
Cholesterol alters the distribution of SNARE and viral fusion proteins in membranes
Cholesterol changes the penetration of fusion peptides in membranes
Cholesterol alters intrinsic membrane curvature and bending in membrane fusion
Cholesterol alters the lifetime of hemifusion intermediates in membrane fusion
Acknowledgments
This work was supported by NIH grants P01 GM72694 and R01 AI30557 and research program grant RGP0055/2015 from the Human Frontier Science Program. We apologize to all those authors whose work could not be discussed owing to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aeffner S, Reusch T, Weinhausen B, Salditt T. Energetics of stalk intermediates in membrane fusion are controlled by lipid composition. Proc Natl Acad Sci U S A. 2012;109:E1609–1618. doi: 10.1073/pnas.1119442109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacia K, Schuette CG, Kahya N, Jahn R, Schwille P. SNAREs prefer liquid-disordered over “raft” (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J Biol Chem. 2004;279:37951–37955. doi: 10.1074/jbc.M407020200. [DOI] [PubMed] [Google Scholar]
- Baier CJ, Fantini J, Barrantes FJ. Disclosure of cholesterol recognition motifs in transmembrane domains of the human nicotinic acetylcholine receptor. Scientific reports. 2011;1:69. doi: 10.1038/srep00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett PJ, Song Y, Van Horn WD, Hustedt EJ, Schafer JM, Hadziselimovic A, Beel AJ, Sanders CR. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science. 2012;336:1168–1171. doi: 10.1126/science.1219988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- Bennett MP, Mitchell DC. Regulation of membrane proteins by dietary lipids: effects of cholesterol and docosahexaenoic acid acyl chain-containing phospholipids on rhodopsin stability and function. Biophysical journal. 2008;95:1206–1216. doi: 10.1529/biophysj.107.122788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Yin SR, Blank PS, Zimmerberg J. Cholesterol promotes hemifusion and pore widening in membrane fusion induced by influenza hemagglutinin. J Gen Physiol. 2008;131:503–513. doi: 10.1085/jgp.200709932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R, Clague MJ, Durell SR, Epand RM. Membrane fusion. Chem Rev. 2003;103:53–69. doi: 10.1021/cr000036+. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci U S A. 2001;98:5619–5624. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SS, Yang P, Ke PY, Li HF, Chan WE, Chang DK, Chuang CK, Tsai Y, Huang SC. Identification of the LWYIK motif located in the human immunodeficiency virus type 1 transmembrane gp41 protein as a distinct determinant for viral infection. Journal of virology. 2009;83:870–883. doi: 10.1128/JVI.01088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Rand RP. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys J. 1997;73:267–276. doi: 10.1016/S0006-3495(97)78067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiantia S, Schwille P, Klymchenko AS, London E. Asymmetric GUVs prepared by MbetaCD-mediated lipid exchange: an FCS study. Biophys J. 2011;100:L1–3. doi: 10.1016/j.bpj.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward MA, Rogasevskaia T, Brandman DM, Khosravani H, Nava P, Atkinson JK, Coorssen JR. Specific lipids supply critical negative spontaneous curvature–an essential component of native Ca2+-triggered membrane fusion. Biophys J. 2008;94:3976–3986. doi: 10.1529/biophysj.107.123984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward MA, Rogasevskaia T, Hofgen J, Bau J, Coorssen JR. Cholesterol facilitates the native mechanism of Ca2+-triggered membrane fusion. J Cell Sci. 2005;118:4833–4848. doi: 10.1242/jcs.02601. [DOI] [PubMed] [Google Scholar]
- Collins MD, Keller SL. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc Natl Acad Sci U S A. 2008;105:124–128. doi: 10.1073/pnas.0702970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Kiessling V, Tamm LK. Measuring lipid asymmetry in planar supported bilayers by fluorescence interference contrast microscopy. Langmuir. 2005;21:1377–1388. doi: 10.1021/la047654w. [DOI] [PubMed] [Google Scholar]
- Crane JM, Tamm LK. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys J. 2004;86:2965–2979. doi: 10.1016/S0006-3495(04)74347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Tamm LK. Fluorescence microscopy to study domains in supported lipid bilayers. Methods Mol Biol. 2007;400:481–488. doi: 10.1007/978-1-59745-519-0_32. [DOI] [PubMed] [Google Scholar]
- de Vries M, Herrmann A, Veit M. A cholesterol consensus motif is required for efficient intracellular transport and raft association of a group 2 HA from influenza virus. The Biochemical journal. 2015;465:305–314. doi: 10.1042/BJ20141114. [DOI] [PubMed] [Google Scholar]
- Destainville N, Schmidt TH, Lang T. Where Biology Meets Physics-A Converging View on Membrane Microdomain Dynamics. Curr Top Membr. 2016;77:27–65. doi: 10.1016/bs.ctm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Domanska MK, Dunning RA, Dryden KA, Zawada KE, Yeager M, Kasson PM. Hemagglutinin Spatial Distribution Shifts in Response to Cholesterol in the Influenza Viral Envelope. Biophys J. 2015;109:1917–1924. doi: 10.1016/j.bpj.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanska MK, Wrona D, Kasson PM. Multiphasic effects of cholesterol on influenza fusion kinetics reflect multiple mechanistic roles. Biophys J. 2013;105:1383–1387. doi: 10.1016/j.bpj.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayake EV, Fu R, Cross TA. Structural Influences: Cholesterol, Drug, and Proton Binding to Full-Length Influenza A M2 Protein. Biophysical journal. 2016;110:1391–1399. doi: 10.1016/j.bpj.2015.11.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrich C, Rentero C, Hierro A, Grewal T. Role of cholesterol in SNARE-mediated trafficking on intracellular membranes. J Cell Sci. 2015;128:1071–1081. doi: 10.1242/jcs.164459. [DOI] [PubMed] [Google Scholar]
- Epand RM. Fusion peptides and the mechanism of viral fusion. Biochim Biophys Acta. 2003;1614:116–121. doi: 10.1016/s0005-2736(03)00169-x. [DOI] [PubMed] [Google Scholar]
- Epand RM. Proteins and cholesterol-rich domains. Biochimica et biophysica acta. 2008;1778:1576–1582. doi: 10.1016/j.bbamem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Evans E, Rawicz W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys Rev Lett. 1990;64:2094–2097. doi: 10.1103/PhysRevLett.64.2094. [DOI] [PubMed] [Google Scholar]
- Falck E, Patra M, Karttunen M, Hyvonen MT, Vattulainen I. Impact of cholesterol on voids in phospholipid membranes. The Journal of chemical physics. 2004;121:12676–12689. doi: 10.1063/1.1824033. [DOI] [PubMed] [Google Scholar]
- Fantini J, Barrantes FJ. How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Frontiers in physiology. 2013;4:31. doi: 10.3389/fphys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J, Yahi N. Molecular basis for the glycosphingolipid-binding specificity of alpha-synuclein: key role of tyrosine 39 in membrane insertion. Journal of molecular biology. 2011;408:654–669. doi: 10.1016/j.jmb.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Feigenson GW. Phase behavior of lipid mixtures. Nat Chem Biol. 2006;2:560–563. doi: 10.1038/nchembio1106-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkenstaedt-Quinn SA, Gruba SM, Haynes CL. Variations in Fusion Pore Formation in Cholesterol-Treated Platelets. Biophys J. 2016;110:922–929. doi: 10.1016/j.bpj.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed EO. HIV-1 assembly, release and maturation. Nat Rev Microbiol. 2015;13:484–496. doi: 10.1038/nrmicro3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Chiantia S, Schwille P. Effect of line tension on the lateral organization of lipid membranes. J Biol Chem. 2007;282:33537–33544. doi: 10.1074/jbc.M706162200. [DOI] [PubMed] [Google Scholar]
- Garg S, Ruhe J, Ludtke K, Jordan R, Naumann CA. Domain registration in raft-mimicking lipid mixtures studied using polymer-tethered lipid bilayers. Biophys J. 2007;92:1263–1270. doi: 10.1529/biophysj.106.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, White JG, Haynes CL. Critical role of membrane cholesterol in exocytosis revealed by single platelet study. ACS Chem Biol. 2010;5:819–828. doi: 10.1021/cb100130b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil C, Soler-Jover A, Blasi J, Aguilera J. Synaptic proteins and SNARE complexes are localized in lipid rafts from rat brain synaptosomes. Biochem Biophys Res Commun. 2005;329:117–124. doi: 10.1016/j.bbrc.2005.01.111. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Burger K, Fahrenholz F. Cholesterol as modulator of receptor function. Biochemistry. 1997;36:10959–10974. doi: 10.1021/bi963138w. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. Cholesterol as stabilizer of the oxytocin receptor. Bba-Biomembranes. 2002;1564:384–392. doi: 10.1016/s0005-2736(02)00475-3. [DOI] [PubMed] [Google Scholar]
- Graham DR, Chertova E, Hilburn JM, Arthur LO, Hildreth JE. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J Virol. 2003;77:8237–8248. doi: 10.1128/JVI.77.15.8237-8248.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruba SM, Koseoglu S, Meyer AF, Meyer BM, Maurer-Jones MA, Haynes CL. Platelet membrane variations and their effects on delta-granule secretion kinetics and aggregation spreading among different species. Biochim Biophys Acta. 2015;1848:1609–1618. doi: 10.1016/j.bbamem.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P, Stevens RC. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M, Bogan JS. Cholesterol regulates glucose-stimulated insulin secretion through phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2009;284:29489–29498. doi: 10.1074/jbc.M109.038034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heftberger P, Kollmitzer B, Rieder AA, Amenitsch H, Pabst G. In situ determination of structure and fluctuations of coexisting fluid membrane domains. Biophys J. 2015;108:854–862. doi: 10.1016/j.bpj.2014.11.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsen JH, Mouritsen OG, Zuckermann MJ. Theory of Thermal Anomalies in the Specific-Heat of Lipid Bilayers Containing Cholesterol. Biophysical Journal. 1989;56:661–667. doi: 10.1016/S0006-3495(89)82713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K, Mouritsen OG, Anderson RGW. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- Jafurulla M, Tiwari S, Chattopadhyay A. Identification of cholesterol recognition amino acid consensus (CRAC) motif in G-protein coupled receptors. Biochem Biophys Res Commun. 2011;404:569–573. doi: 10.1016/j.bbrc.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Jamin N, Neumann JM, Ostuni MA, Vu TK, Yao ZX, Murail S, Robert JC, Giatzakis C, Papadopoulos V, Lacapere JJ. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Molecular endocrinology. 2005;19:588–594. doi: 10.1210/me.2004-0308. [DOI] [PubMed] [Google Scholar]
- Julicher F, Lipowsky R. Domain-induced budding of vesicles. Phys Rev Lett. 1993;70:2964–2967. doi: 10.1103/PhysRevLett.70.2964. [DOI] [PubMed] [Google Scholar]
- Kahya N, Scherfeld D, Bacia K, Poolman B, Schwille P. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J Biol Chem. 2003;278:28109–28115. doi: 10.1074/jbc.M302969200. [DOI] [PubMed] [Google Scholar]
- Kiessling V, Crane JM, Tamm LK. Transbilayer effects of raft-like lipid domains in asymmetric planar bilayers measured by single molecule tracking. Biophys J. 2006;91:3313–3326. doi: 10.1529/biophysj.106.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling V, Wan C, Tamm LK. Domain coupling in asymmetric lipid bilayers. Biochim Biophys Acta. 2009;1788:64–71. doi: 10.1016/j.bbamem.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling V, Yang ST, Tamm LK. Supported Lipid Bilayers as Models for Studying Membrane Domains. Lipid Domains. 2015;75:1–23. doi: 10.1016/bs.ctm.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Knowles MK, Barg S, Wan L, Midorikawa M, Chen X, Almers W. Single secretory granules of live cells recruit syntaxin-1 and synaptosomal associated protein 25 (SNAP-25) in large copy numbers. Proc Natl Acad Sci U S A. 2010;107:20810–20815. doi: 10.1073/pnas.1014840107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseoglu S, Love SA, Haynes CL. Cholesterol effects on vesicle pools in chromaffin cells revealed by carbon-fiber microelectrode amperometry. Anal Bioanal Chem. 2011;400:2963–2971. doi: 10.1007/s00216-011-5002-7. [DOI] [PubMed] [Google Scholar]
- Kreutzberger AJ, Kiessling V, Tamm LK. High cholesterol obviates a prolonged hemifusion intermediate in fast SNARE-mediated membrane fusion. Biophys J. 2015;109:319–329. doi: 10.1016/j.bpj.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruit JK, Wijesekara N, Fox JE, Dai XQ, Brunham LR, Searle GJ, Morgan GP, Costin AJ, Tang R, Bhattacharjee A, Johnson JD, Light PE, Marsh BJ, Macdonald PE, Verchere CB, Hayden MR. Islet cholesterol accumulation due to loss of ABCA1 leads to impaired exocytosis of insulin granules. Diabetes. 2011;60:3186–3196. doi: 10.2337/db11-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AL, Li YL, Tamm LK. Interplay of Proteins and Lipids in Virus Entry by Membrane Fusion. Protein-Lipid Interactions: From Membrane Domains to Cellular Networks. 2005:279–303. [Google Scholar]
- Lai AL, Moorthy AE, Li Y, Tamm LK. Fusion activity of HIV gp41 fusion domain is related to its secondary structure and depth of membrane insertion in a cholesterol-dependent fashion. J Mol Biol. 2012;418:3–15. doi: 10.1016/j.jmb.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T. SNARE proteins and ‘membrane rafts’. J Physiol. 2007;585:693–698. doi: 10.1113/jphysiol.2007.134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DE, Lew MG, Woodbury DJ. Vesicle fusion to planar membranes is enhanced by cholesterol and low temperature. Chem Phys Lipids. 2013;166:45–54. doi: 10.1016/j.chemphyslip.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1267–1272. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Dawidowski D, Ellena JF, Tamm LK, Cafiso DS. The SNARE motif of synaptobrevin exhibits an aqueous-interfacial partitioning that is modulated by membrane curvature. Biochemistry-Us. 2014;53:1485–1494. doi: 10.1021/bi401638u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Cimakasky LM, Hampton R, Nguyen DH, Hildreth JE. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res Hum Retroviruses. 2001;17:1009–1019. doi: 10.1089/088922201300343690. [DOI] [PubMed] [Google Scholar]
- Lin Q, London E. Preparation of artificial plasma membrane mimicking vesicles with lipid asymmetry. PLoS One. 2014;9:e87903. doi: 10.1371/journal.pone.0087903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetti A, Fratangeli A, Taverna E, Valnegri P, Francolini M, Cappello V, Matteoli M, Passafaro M, Rosa P. Cholesterol reduction impairs exocytosis of synaptic vesicles. J Cell Sci. 2010;123:595–605. doi: 10.1242/jcs.060681. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid Rafts As a Membrane-Organizing Principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Manes S, del Real G, Martinez AC. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- Milovanovic D, Honigmann A, Koike S, Gottfert F, Pahler G, Junius M, Mullar S, Diederichsen U, Janshoff A, Grubmuller H, Risselada HJ, Eggeling C, Hell SW, van den Bogaart G, Jahn R. Hydrophobic mismatch sorts SNARE proteins into distinct membrane domains. Nat Commun. 2015;6:5984. doi: 10.1038/ncomms6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DH, Tamm LK. Clustering of syntaxin-1A in model membranes is modulated by phosphatidylinositol 4,5-bisphosphate and cholesterol. Biochemistry-Us. 2009;48:4617–4625. doi: 10.1021/bi9003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DH, Tamm LK. Molecular mechanism of cholesterol- and polyphosphoinositide-mediated syntaxin clustering. Biochemistry-Us. 2011;50:9014–9022. doi: 10.1021/bi201307u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu SL, Mitchell DC, Litman BJ. Manipulation of cholesterol levels in rod disk membranes by methyl-beta-cyclodextrin: effects on receptor activation. The Journal of biological chemistry. 2002;277:20139–20145. doi: 10.1074/jbc.M200594200. [DOI] [PubMed] [Google Scholar]
- Oddi S, Dainese E, Fezza F, Lanuti M, Barcaroli D, De Laurenzi V, Centonze D, Maccarrone M. Functional characterization of putative cholesterol binding sequence (CRAC) in human type-1 cannabinoid receptor. J Neurochem. 2011;116:858–865. doi: 10.1111/j.1471-4159.2010.07041.x. [DOI] [PubMed] [Google Scholar]
- Paila YD, Chattopadhyay A. Membrane cholesterol in the function and organization of G-protein coupled receptors. Sub-cellular biochemistry. 2010;51:439–466. doi: 10.1007/978-90-481-8622-8_16. [DOI] [PubMed] [Google Scholar]
- Pan J, Mills TT, Tristram-Nagle S, Nagle JF. Cholesterol perturbs lipid bilayers nonuniversally. Phys Rev Lett. 2008;100:198103. doi: 10.1103/PhysRevLett.100.198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Tristram-Nagle S, Nagle JF. Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;80:021931. doi: 10.1103/PhysRevE.80.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman JS, Jing X, Leser GP, Lamb RA. Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell. 2010;142:902–913. doi: 10.1016/j.cell.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. The principle of membrane fusion in the cell (Nobel lecture) Angew Chem Int Ed Engl. 2014;53:12676–12694. doi: 10.1002/anie.201402380. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Smith BA, McConnell HM. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979;76:15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram MB, Thompson TE. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry-Us. 1990;29:10676–10684. doi: 10.1021/bi00499a015. [DOI] [PubMed] [Google Scholar]
- Saxena R, Chattopadhyay A. Membrane cholesterol stabilizes the human serotonin(1A) receptor. Biochim Biophys Acta. 2012;1818:2936–2942. doi: 10.1016/j.bbamem.2012.07.032. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Roth MG, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Grzybek M, Schwarzmann G, Mueller V, Honigmann A, Belov VN, Eggeling C, Coskun U, Simons K, Schwille P. Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochim Biophys Acta. 2012;1818:1777–1784. doi: 10.1016/j.bbamem.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Edidin MA. Membranes are not just rafts. Chem Phys Lipids. 2006;144:1–3. doi: 10.1016/j.chemphyslip.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Sieber JJ, Willig KI, Kutzner C, Gerding-Reimers C, Harke B, Donnert G, Rammner B, Eggeling C, Hell SW, Grubmuller H, Lang T. Anatomy and dynamics of a supramolecular membrane protein cluster. Science. 2007;317:1072–1076. doi: 10.1126/science.1141727. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Smondyrev AM, Berkowitz ML. Structure of dipalmitoylphosphatidylcholine/cholesterol bilayer at low and high cholesterol concentrations: Molecular dynamics simulation. Biophysical Journal. 1999;77:2075–2089. doi: 10.1016/S0006-3495(99)77049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubias O, Gawrisch K. The role of the lipid matrix for structure and function of the GPCR rhodopsin. Bba-Biomembranes. 2012;1818:234–240. doi: 10.1016/j.bbamem.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton BS, Warner JM, Wu Z, Nikolaus J, Wei G, Wagnon E, Baddeley D, Karatekin E, O’Shaughnessy B. Cholesterol increases the openness of SNARE-mediated flickering fusion pores. Biophys J. 2016;110:1538–1550. doi: 10.1016/j.bpj.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturek JM, Castle JD, Trace AP, Page LC, Castle AM, Evans-Molina C, Parks JS, Mirmira RG, Hedrick CC. An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic beta cells. J Clin Invest. 2010;120:2575–2589. doi: 10.1172/JCI41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Whittaker GR. Role for influenza virus envelope cholesterol in virus entry and infection. J Virol. 2003;77:12543–12551. doi: 10.1128/JVI.77.23.12543-12551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm LK, Crane J, Kiessling V. Membrane fusion: a structural perspective on the interplay of lipids and proteins. Curr Opin Struct Biol. 2003;13:453–466. doi: 10.1016/s0959-440x(03)00107-6. [DOI] [PubMed] [Google Scholar]
- Tamm LK, Han X, Li Y, Lai AL. Structure and function of membrane fusion peptides. Biopolymers. 2002;66:249–260. doi: 10.1002/bip.10261. [DOI] [PubMed] [Google Scholar]
- Tamm LK, Lee J, Liang B. Capturing glimpses of an elusive HIV gp41 prehairpin fusion intermediate. Structure. 2014;22:1225–1226. doi: 10.1016/j.str.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Borbat PP, Freed JH, Shin YK. A scissors mechanism for stimulation of SNARE-mediated lipid mixing by cholesterol. Proc Natl Acad Sci U S A. 2009;106:5141–5146. doi: 10.1073/pnas.0813138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaart G, Jahn R. Counting the SNAREs needed for membrane fusion. J Mol Cell Biol. 2011;3:204–205. doi: 10.1093/jmcb/mjr004. [DOI] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Bio. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Vishwanathan SA, Thomas A, Brasseur R, Epand RF, Hunter E, Epand RM. Hydrophobic substitutions in the first residue of the CRAC segment of the gp41 protein of HIV. Biochemistry. 2008;47:124–130. doi: 10.1021/bi7018892. [DOI] [PubMed] [Google Scholar]
- Waheed AA, Freed EO. Lipids and membrane microdomains in HIV-1 replication. Virus Res. 2009;143:162–176. doi: 10.1016/j.virusres.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C, Kiessling V, Cafiso DS, Tamm LK. Partitioning of synaptotagmin I C2 domains between liquid-ordered and liquid-disordered inner leaflet lipid phases. Biochemistry-Us. 2011;50:2478–2485. doi: 10.1021/bi101864k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C, Kiessling V, Tamm LK. Coupling of cholesterol-rich lipid phases in asymmetric bilayers. Biochemistry-Us. 2008;47:2190–2198. doi: 10.1021/bi7021552. [DOI] [PubMed] [Google Scholar]
- Wang N, Kwan C, Gong X, de Chaves EP, Tse A, Tse FW. Influence of cholesterol on catecholamine release from the fusion pore of large dense core chromaffin granules. J Neurosci. 2010;30:3904–3911. doi: 10.1523/JNEUROSCI.4000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol. 2007;579:413–429. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Huang HW. Observation of a membrane fusion intermediate structure. Science. 2002;297:1877–1879. doi: 10.1126/science.1074354. [DOI] [PubMed] [Google Scholar]
- Yang ST, Kiessling V, Simmons JA, White JM, Tamm LK. HIV gp41-mediated membrane fusion occurs at edges of cholesterol-rich lipid domains. Nat Chem Biol. 2015;11:424–431. doi: 10.1038/nchembio.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ST, Kiessling V, Tamm LK. Line tension at lipid phase boundaries as driving force for HIV fusion peptide-mediated fusion. Nat Commun. 2016;7:11401. doi: 10.1038/ncomms11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xue R, Ong WY, Chen P. Roles of cholesterol in vesicle fusion and motion. Biophys J. 2009;97:1371–1380. doi: 10.1016/j.bpj.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J, Vogel SS, Chernomordik LV. Mechanisms of membrane fusion. Annu Rev Biophys Biomol Struct. 1993;22:433–466. doi: 10.1146/annurev.bb.22.060193.002245. [DOI] [PubMed] [Google Scholar]


