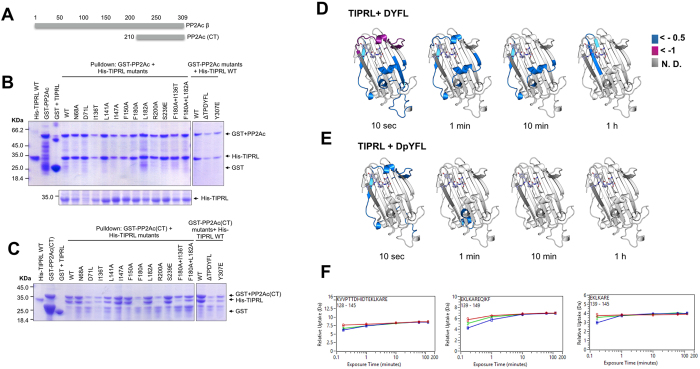
Figure 4. Validation of the PP2A C-terminus binding site on TIPRL by mutagenesis/pulldown and HDX/MS.
(A) Schematic representation of the PP2A constructs used for GST pulldown. (B) GST-pulldown assays of mutated TIPRL. GST-tagged, full length PP2Acβ and His-tagged, full length TIPRL or its mutated versions were co-expressed in E. coli and the lysates were incubated with glutathione-sepharose beads. The right panel shows WT His-TIPRL combined with a truncation mutation (ΔTPDYFL) and a phosphomimetic mutation of PP2Ac (Y307E). No substantial effects were observed for any of the mutations. The lower panel depicts the soluble lysates, showing reduced expression levels for D71L. (C) The TIPRL mutants in (B) were assayed for their interaction with PP2Ac (residues 210–309). The mutants R200A and F180A + I136T show substantial loss of binding to PP2Ac. (D,E) Structural representation of the relative deuterium incorporation (in comparison with apo protein) from HDX-MS analysis of full length, his-tagged TIPRL, in the presence of a synthetic tetrapeptide which mimics the PP2A C-terminus, unmodified (D) or phosphorylated (E). The bottom side of TIPRL is shown in cartoon representation and the DYFL peptide is represented as blue sticks. The relative deuterium exchange is represented as follows: Blue: 0 to −0.5 Da, Purple: 0.5 to −1.0 Da, Dark grey: not determined. In both cases, only negative values were observed, indicating either a gain of structure or reduced solvent exposure compared to the control sample with no bound peptide. The 2 hour time points are identical to the respective 1 hour timepoint and therefore are not depicted in the figure. (F) Representative deuterium incorporation plots of peptides from the TIPRL connector loop, in the apo protein (red) or in the presence of a synthetic tetrapeptide which mimics the PP2A C-terminus, unmodified (blue) or phosphorylated (green).